Eptapiron
Prijeđi na navigaciju
Prijeđi na pretragu
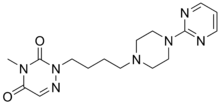
| |||
| (IUPAC) ime | |||
|---|---|---|---|
| 4-metil-2-[4-(4-pirimidin-2-ilpiperazin-1-il)butil]-1,2,4-triazin-3,5-dion | |||
| Klinički podaci | |||
| Identifikatori | |||
| CAS broj | 179756-85-5 | ||
| ATC kod | nije dodeljen | ||
| PubChem[1][2] | 208928 | ||
| ChemSpider[3] | 181024 | ||
| UNII | 3M824XRO8N | ||
| Hemijski podaci | |||
| Formula | C16H23N7O2 | ||
| Mol. masa | 345,40 g/mol | ||
| SMILES | eMolekuli & PubHem | ||
| |||
| Farmakokinetički podaci | |||
| Poluvreme eliminacije | ~2 sata | ||
| Farmakoinformacioni podaci | |||
| Trudnoća | ? | ||
| Pravni status | Uncontrolled | ||
| Način primene | Oralno | ||
Eptapiron (F-11,440) je veoma potentan i visoko selektivan pun agonist 5-HT1A receptora iz azapironske klase.[4][5] Njegov afinitet za 5-HT1A receptor je 4.8 nM (Ki) ili 8.33 (pKi), a njegova intrinsična aktivnost je 100%, i.e. jednaka je sa serotoninom.[4]
Eptapiron i srodna jedinjenja, poput F-13,640 i F-15,599, su razvijena jer se smatralo da je visoka efikasnost aktivacije receptora neohodna da bi se proizvela maksimalna terapeutska korist stimulacije 5-HT1A receptora.[4][5][6][7]
References[uredi | uredi kod]
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519.
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining”. J Cheminform 2 (1): 3. DOI:10.1186/1758-2946-2-3. PMID 20331846.
- ↑ 4,0 4,1 4,2 Koek W, Patoiseau JF, Assié MB, et al. (October 1998). „F 11440, a potent, selective, high efficacy 5-HT1A receptor agonist with marked anxiolytic and antidepressant potential”. The Journal of Pharmacology and Experimental Therapeutics 287 (1): 266–83. PMID 9765347.
- ↑ 5,0 5,1 Prinssen EP, Colpaert FC, Koek W (October 2002). „5-HT1A receptor activation and anti-cataleptic effects: high-efficacy agonists maximally inhibit haloperidol-induced catalepsy”. European Journal of Pharmacology 453 (2-3): 217–21. DOI:10.1016/S0014-2999(02)02430-5. PMID 12398907.
- ↑ Koek W, Vacher B, Cosi C, et al. (May 2001). „5-HT1A receptor activation and antidepressant-like effects: F 13714 has high efficacy and marked antidepressant potential”. European Journal of Pharmacology 420 (2-3): 103–12. DOI:10.1016/S0014-2999(01)01011-1. PMID 11408031.
- ↑ Maurel JL, Autin JM, Funes P, Newman-Tancredi A, Colpaert F, Vacher B (October 2007). „High-efficacy 5-HT1A agonists for antidepressant treatment: a renewed opportunity”. Journal of Medicinal Chemistry 50 (20): 5024–33. DOI:10.1021/jm070714l. PMID 17803293.