2-Metil-5-hidroksitriptamin
Prijeđi na navigaciju
Prijeđi na pretragu
| |||
| |||
| (IUPAC) ime | |||
|---|---|---|---|
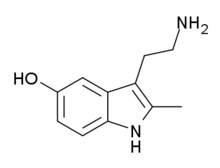
| 3-(2-aminoethyl)-2-methyl-1H-indol-5-ol | |||
| Klinički podaci | |||
| Identifikatori | |||
| CAS broj | 78263-90-8 | ||
| ATC kod | ? | ||
| PubChem[1][2] | 1574 | ||
| ChemSpider[3] | 1518 | ||
| KEGG[4] | C13665 | ||
| ChEMBL[5] | CHEMBL266591 | ||
| Hemijski podaci | |||
| Formula | C11H14N2O | ||
| Mol. masa | 190.242 g/mol | ||
| SMILES | eMolekuli & PubHem | ||
| |||
| Farmakoinformacioni podaci | |||
| Trudnoća | ? | ||
| Pravni status | |||
2-Metil-5-hidroksitriptamin (2-Metilserotonin, 2-Metil-5-HT) je triptaminski derivat koji je blisko srodan neurotransmiteru serotonin. On deluje kao umereno selektivan agonist 5-HT3 receptora.[6][7][8]
Vidi još[uredi | uredi kod]
Literatura[uredi | uredi kod]
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519.
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining”. J Cheminform 2 (1): 3. DOI:10.1186/1758-2946-2-3. PMID 20331846.
- ↑ Joanne Wixon, Douglas Kell (2000). „Website Review: The Kyoto Encyclopedia of Genes and Genomes — KEGG”. Yeast 17 (1): 48–55. DOI:10.1002/(SICI)1097-0061(200004)17:1<48::AID-YEA2>3.0.CO;2-H.
- ↑ Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B, Overington JP. (2012). „ChEMBL: a large-scale bioactivity database for drug discovery”. Nucleic Acids Res 40 (Database issue): D1100-7. DOI:10.1093/nar/gkr777. PMID 21948594.
- ↑ Elz S, Zimmermann H, Rehse K. (1993). „Selectivity of sterically fixed tryptamine and 5-methoxytryptamine derivatives for serotonin receptor subtypes, II: Structure-activity relationships and in vitro pharmacology of N-alkyl- and N,N-dialkyl-3- indolylbicyclo-[2.2.1]-heptane-2-amines.”. Arch Pharm (Weinheim). 326 (11): 893–899. DOI:10.1002/ardp.19933261110. PMID 8274071.
- ↑ Craig DA, Eglen RM, Walsh LK, Perkins LA, Whiting RL, Clarke DE. (1990). „5-Methoxytryptamine and 2-methyl-5-hydroxytryptamine-induced desensitization as a discriminative tool for the 5-HT3 and putative 5-HT4 receptors in guinea pig ileum.”. Naunyn Schmiedebergs Arch Pharmacol. 342 (1): 9–16. PMID 2402303.
- ↑ Glennon RA, Dukat M, Westkaemper RB (01. 01. 2000.). „Serotonin Receptor Subtypes and Ligands”. American College of Neurophyscopharmacology. Pristupljeno 11. 04. 2008.