Roksatidin
Prijeđi na navigaciju
Prijeđi na pretragu
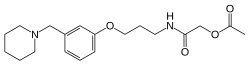
| |||
| (IUPAC) ime | |||
|---|---|---|---|
| 2-okso-2-(3-[3-(piperidin-1-ilmetil)fenoksi]propilamino)etil acetat | |||
| Klinički podaci | |||
| Identifikatori | |||
| CAS broj | 78628-28-1 | ||
| ATC kod | A02BA06 | ||
| PubChem[1][2] | 5105 | ||
| DrugBank | DB08806 | ||
| ChemSpider[3] | 4926 | ||
| UNII | IV9VHT3YUM | ||
| KEGG[4] | D08495 | ||
| ChEMBL[5] | CHEMBL46102 | ||
| Hemijski podaci | |||
| Formula | C19H28N2O4 | ||
| Mol. masa | 348,437 g/mol | ||
| SMILES | eMolekuli & PubHem | ||
| |||
| Farmakokinetički podaci | |||
| Bioraspoloživost | 80–90% | ||
| Vezivanje za proteine plazme | 5–7% | ||
| Metabolizam | Hepatička deacetilacija Minje učešće CYP2D6 i CYP2A6 | ||
| Poluvreme eliminacije | 5–7 sata | ||
| Izlučivanje | Renalno | ||
| Farmakoinformacioni podaci | |||
| Trudnoća | ? | ||
| Pravni status | |||
| Način primene | Oralno | ||
Roksatidin acetat je specifični i kompetitivni antagonist H2 receptora. Antisekretorno dejstvo roksatidin acetata je posredovano njegovim glavnim metabolitom, roksatidinom.

Farmakodinamičke studije su pokazale da je 150 mg roksatidin acetata optimalno u suzbijanju sekrecije želudačne kiseline.[6]
Reference[uredi | uredi kod]
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519.
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining”. J Cheminform 2 (1): 3. DOI:10.1186/1758-2946-2-3. PMID 20331846.
- ↑ Joanne Wixon, Douglas Kell (2000). „Website Review: The Kyoto Encyclopedia of Genes and Genomes — KEGG”. Yeast 17 (1): 48–55. DOI:10.1002/(SICI)1097-0061(200004)17:1<48::AID-YEA2>3.0.CO;2-H.
- ↑ Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B, Overington JP. (2012). „ChEMBL: a large-scale bioactivity database for drug discovery”. Nucleic Acids Res 40 (Database issue): D1100-7. DOI:10.1093/nar/gkr777. PMID 21948594.
- ↑ Murdoch D, McTavish D (1991). „Roxatidine acetate. A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic potential in peptic ulcer disease and related disorders”. Drugs 42 (2): 240-260. PMID 1717223.