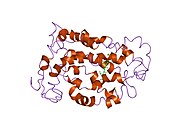
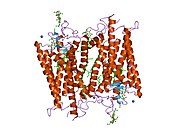
Rodopsin
| edit |
| Rodopsin (opsin 2, štap pigment) (retinitis pigmentosa 4, autosomno dominantan) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
 Senzorni rodopsin II (duginih boja) smešten u lipidnom dvosloju (glave crveno i repovi plavo) sa transducinom ispod njega. Gtα je obojen crveno, Gtβ plavo, i Gtγ žuto. GDP molekul je vezan u Gtα-podjedinici i kovalento vezani retinal (crno ) je u rodopsinu. N-terminus rodopsina je crven i C-terminus je plav. Pretpostavljeno ankerisanje transducina za membranu je prikazano u crnoj boji. | |||||||||||
| Dostupne strukture | |||||||||||
| 1eds, 1edx, 1f88, 1gzm, 1hzx, 1jfp, 1l9h, 1ln6, 1u19, 2g87, 2hpy, 2i35, 2i36, 2i37 | |||||||||||
| Identifikatori | |||||||||||
| Simboli | RHO; MGC138309; MGC138311; OPN2; RP4 | ||||||||||
| Vanjski ID | OMIM: 180380 MGI: 97914 HomoloGene: 68068 GeneCards: RHO Gene | ||||||||||
| |||||||||||
| Pregled RNK izražavanja | |||||||||||
 | |||||||||||
 | |||||||||||
| podaci | |||||||||||
| Ortolozi | |||||||||||
| Vrsta | Čovek | Miš | |||||||||
| Entrez | 6010 | 212541 | |||||||||
| Ensembl | ENSG00000163914 | ENSMUSG00000030324 | |||||||||
| UniProt | P08100 | Q8K0D8 | |||||||||
| RefSeq (mRNA) | NM_000539 | NM_145383 | |||||||||
| RefSeq (protein) | NP_000530 | NP_663358 | |||||||||
| Lokacija (UCSC) | Chr 3: 130.73 - 130.74 Mb | Chr 6: 115.9 - 115.9 Mb | |||||||||
| PubMed pretraga | [1] | [2] | |||||||||
Rodopsin, mrežnjačin purpur, je pigment retine koji je odgovoran za formiranje fotoreceptorskih ćelija, i za prve događaje u percepciji svetlosti. Rodopsini pripadaju familiji G-protein spregnutih receptora i ekstremno su senzitivni na svetlost, što omogućava vid u slabo-osvetljenoj sredini.[1] Exposed to light, the pigment immediately photobleaches, and it takes about 30 minutes[2] to regenerate fully in humans.
Struktura[uredi | uredi kod]
Rodopsin se sastoji od proteinskog dela opsina i reverzibilno kovalentno vezanog kofaktor, retinala. Opsin, svežanj sedam transmembranskih heliksa međusobno povezanih proteinskim petljama, vezuje retinal (fotoreaktivni hromofor), koji je lociran u centralnoj šupljini na lizinskom ostatku sedmog heliksa. Retinal zauzima horizontalan položaj u odnosu na membranu. Svaki spoljašnji segment diska sadrži hiljade vizuelnih molekula pigmenta. Oko polovine opsina je unutar lipidnog dvosloja. Retinal nastaje u retini iz Vitamina A, dijetarnog beta-karotena. Izomerizacija 11-cis-retinala u sve-trans-retinal pod uticajem svetlosti indukuje konformacionu promenu (beljenje) u opsina, koja se nastavlja sa metarodopsinom II, koji aktivira vezani G protein transducin, i započinje kaskadu sekundarnih glasnika.[2][3] [4]
Rodopsin štapića najsnažnije apsorbuje zeleno-plavu svetlost, i zato izgleda crvenkasto-ljubičasto. On se naziva "vizuelno ljubičasto". Rodopsin je odgovoran za monohromatski vid u tami.[2]

Nekoliko blisko srodnih opsina postoji. Oni se razlikuju u nekoliko aminokiselina, i konsekventno u talasnoj dužini svetlosti koju najjače apsorbuju. Kod čoveka postoje četiri različita opsina pored rodopsina. Fotopsini se nalazi u različitim tipovima koničnih ćelija retine, i oni su baza raspoznavanja boja. Oni imaju maksimume apsorpcije za žuto-zeleno (fotopsin I), zeleno (fotopsin II), i plavo-ljubičasto (fotopsin III) svetlo. Preostali opsin (melanopsin) se nalazi u fotosenzitivnim ganglionskim ćelijama, i apsorbuje najjače plavu svetlost.
Struktura rodopsina je detaljno proučena putem rendgenske strukturne analize kristala rodopsina. Fotoizomerizaciona dinamika je bila istražena putem FTIR spektroskopije i UV/Vis spektroskopije. Prvi fotoprodukt, fotorodopsin, se formira u toku 200 femtosekundi nakon iradijacije, čemu sledi u toku nekoliko pikosekundi drugi, batorodopsin, sa deformisanim sve-trans vezama. Taj intermedijar može da bude zarobljen i studiran na kriogenim temperaturama. Više modela (npr. mehanizam pedala bicikla, hula-tvist mehanizam) pokušava da objasni kako retinalna grupa može da promeni svoju konformaciju bez sudaranja sa okružujućim rodopsin proteinskim džepom.[5][6][7]
Nedavni nalazi indiciraju da rodopsin funkcionače kao monomer, a ne kao dimer, mada je to dugo vremena bila ustaljena paradigma za G-spregnute proteinske receptore.[8]
Reference[uredi | uredi kod]
- ↑ Litmann BJ, Mitchell DC (1996). „Rhodopsin structure and function”. u: Lee AG. Rhodopsin and G-Protein Linked Receptors, Part A (Vol 2, 1996) (2 Vol Set). Greenwich, Conn: JAI Press. str. 1-32. ISBN 978-1-55938-659-3.
- ↑ 2,0 2,1 2,2 Stuart JA, Brige RR (1996). „Characterization of the primary photochemical events in bacteriorhodopsin and rhodopsin”. u: Lee AG. Rhodopsin and G-Protein Linked Receptors, Part A (Vol 2, 1996) (2 Vol Set). Greenwich, Conn: JAI Press. str. 33-140. ISBN 978-1-55938-659-3.
- ↑ Hofmann KP, Heck M (1996). „Light-induced protein-protein interactions on the rod photoreceptor disc membrane”. u: Lee AG. Rhodopsin and G-Protein Linked Receptors, Part A (Vol 2, 1996) (2 Vol Set). Greenwich, Conn: JAI Press. str. 141-198. ISBN 978-1-55938-659-3.
- ↑ Kolb H, Fernandez E, Nelson R, Jones BW (1. 3. 2010.). „Webvision: Photoreceptors”. University of Utah. Arhivirano iz originala na datum 2000-08-16.
- ↑ Nakamichi H, Okada T (June 2006). „Crystallographic analysis of primary visual photochemistry”. Angew. Chem. Int. Ed. Engl. 45 (26): 4270-3. DOI:10.1002/anie.200600595. PMID 16586416.
- ↑ Schreiber M, Sugihara M, Okada T, Buss V (June 2006). „Quantum mechanical studies on the crystallographic model of bathorhodopsin”. Angew. Chem. Int. Ed. Engl. 45 (26): 4274-7. DOI:10.1002/anie.200600585. PMID 16729349.
- ↑ Weingart O (September 2007). „The twisted C11-C12 bond of the rhodopsin chromophore--a photochemical hot spot”. J. Am. Chem. Soc. 129 (35): 10618-9. DOI:10.1021/ja071793t. PMID 17691730.
- ↑ „Monomeric G-Protein-Coupled Receptor as a Functional Unit - Biochemistry (ACS Publications)”.
Literatura[uredi | uredi kod]
- Hofmann KP, Heck M (1996). Lee AG. ur. Rhodopsin and G-Protein Linked Receptors, Part A (Vol 2, 1996) (2 Vol Set). Greenwich, Conn: JAI Press. str. 141-198.
- Stuart JA, Brige RR (1996). Lee AG. ur. Rhodopsin and G-Protein Linked Receptors, Part A (Vol 2, 1996) (2 Vol Set). Greenwich, Conn: JAI Press. str. 33-140.
- Litmann BJ, Mitchell DC (1996). Lee AG. ur. Rhodopsin and G-Protein Linked Receptors, Part A (Vol 2, 1996) (2 Vol Set). Greenwich, Conn: JAI Press. str. 1-32.
- Humphries P, Kenna P, Farrar GJ (1992). „On the molecular genetics of retinitis pigmentosa.”. Science 256 (5058): 804-8. DOI:10.1126/science.1589761. PMID 1589761.
- Edwards SC (1995). „Involvement of cGMP and calcium in the photoresponse in vertebrate photoreceptor cells.”. The Journal of the Florida Medical Association 82 (7): 485-8. PMID 7673885.
- al-Maghtheh M, Gregory C, Inglehearn C, et al. (1993). „Rhodopsin mutations in autosomal dominant retinitis pigmentosa.”. Hum. Mutat. 2 (4): 249-55. DOI:10.1002/humu.1380020403. PMID 8401533.
- Garriga P, Manyosa J (2002). „The eye photoreceptor protein rhodopsin. Structural implications for retinal disease.”. FEBS Lett. 528 (1-3): 17-22. DOI:10.1016/S0014-5793(02)03241-6. PMID 12297272.
- Mendes HF, van der Spuy J, Chapple JP, Cheetham ME (2005). „Mechanisms of cell death in rhodopsin retinitis pigmentosa: implications for therapy.”. Trends in molecular medicine 11 (4): 177-85. DOI:10.1016/j.molmed.2005.02.007. PMID 15823756.
- Inglehearn CF, Keen TJ, Bashir R, et al. (1993). „A completed screen for mutations of the rhodopsin gene in a panel of patients with autosomal dominant retinitis pigmentosa.”. Hum. Mol. Genet. 1 (1): 41-5. DOI:10.1093/hmg/1.1.41. PMID 1301135.
- Farrar GJ, Findlay JB, Kumar-Singh R, et al. (1993). „Autosomal dominant retinitis pigmentosa: a novel mutation in the rhodopsin gene in the original 3q linked family.”. Hum. Mol. Genet. 1 (9): 769-71. DOI:10.1093/hmg/1.9.769. PMID 1302614.
- Robinson PR, Cohen GB, Zhukovsky EA, Oprian DD (1992). „Constitutively active mutants of rhodopsin.”. Neuron 9 (4): 719-25. DOI:10.1016/0896-6273(92)90034-B. PMID 1356370.
- Fujiki K, Hotta Y, Hayakawa M, et al. (1992). „Point mutations of rhodopsin gene found in Japanese families with autosomal dominant retinitis pigmentosa (ADRP).”. Jpn. J. Hum. Genet. 37 (2): 125-32. DOI:10.1007/BF01899733. PMID 1391967.
- Olsson JE, Gordon JW, Pawlyk BS, et al. (1992). „Transgenic mice with a rhodopsin mutation (Pro23His): a mouse model of autosomal dominant retinitis pigmentosa.”. Neuron 9 (5): 815-30. DOI:10.1016/0896-6273(92)90236-7. PMID 1418997.
- Andréasson S, Ehinger B, Abrahamson M, Fex G (1993). „A six-generation family with autosomal dominant retinitis pigmentosa and a rhodopsin gene mutation (arginine-135-leucine).”. Ophthalmic paediatrics and genetics 13 (3): 145-53. DOI:10.3109/13816819209046483. PMID 1484692.
- Inglehearn CF, Lester DH, Bashir R, et al. (1992). „Recombination between rhodopsin and locus D3S47 (C17) in rhodopsin retinitis pigmentosa families.”. Am. J. Hum. Genet. 50 (3): 590-7. PMC 1684283. PMID 1539595.
- Fishman GA, Stone EM, Gilbert LD, Sheffield VC (1992). „Ocular findings associated with a rhodopsin gene codon 106 mutation. Glycine-to-arginine change in autosomal dominant retinitis pigmentosa.”. Arch. Ophthalmol. 110 (5): 646-53. PMID 1580841.
- Keen TJ, Inglehearn CF, Lester DH, et al. (1992). „Autosomal dominant retinitis pigmentosa: four new mutations in rhodopsin, one of them in the retinal attachment site.”. Genomics 11 (1): 199-205. DOI:10.1016/0888-7543(91)90119-Y. PMID 1765377.
- Dryja TP, Hahn LB, Cowley GS, et al. (1991). „Mutation spectrum of the rhodopsin gene among patients with autosomal dominant retinitis pigmentosa.”. Proc. Natl. Acad. Sci. U.S.A. 88 (20): 9370-4. DOI:10.1073/pnas.88.20.9370. PMC 52716. PMID 1833777.
- Gal A, Artlich A, Ludwig M, et al. (1992). „Pro-347-Arg mutation of the rhodopsin gene in autosomal dominant retinitis pigmentosa.”. Genomics 11 (2): 468-70. DOI:10.1016/0888-7543(91)90159-C. PMID 1840561.
- Sung CH, Davenport CM, Hennessey JC, et al. (1991). „Rhodopsin mutations in autosomal dominant retinitis pigmentosa.”. Proc. Natl. Acad. Sci. U.S.A. 88 (15): 6481-5. DOI:10.1073/pnas.88.15.6481. PMC 52109. PMID 1862076.
- Jacobson SG, Kemp CM, Sung CH, Nathans J (1991). „Retinal function and rhodopsin levels in autosomal dominant retinitis pigmentosa with rhodopsin mutations.”. Am. J. Ophthalmol. 112 (3): 256-71. PMID 1882937.
- Sheffield VC, Fishman GA, Beck JS, et al. (1991). „Identification of novel rhodopsin mutations associated with retinitis pigmentosa by GC-clamped denaturing gradient gel electrophoresis.”. Am. J. Hum. Genet. 49 (4): 699-706. PMC 1683182. PMID 1897520.
- Kolb H, Fernandez E, Nelson R, Jones BW (1. 3. 2010.). „Webvision Home Page: The organization of the retina and visual system”. University of Utah.