LY-379,268
Prijeđi na navigaciju
Prijeđi na pretragu
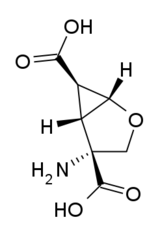
| |||
| (IUPAC) ime | |||
|---|---|---|---|
| (1S,2R,5R,6R)-2-amino-4-oksabiciklo[3.1.0]heksan-2,6-dikarboksilna kiselina | |||
| Klinički podaci | |||
| Identifikatori | |||
| CAS broj | 191471-52-0 | ||
| ATC kod | nije dodeljen | ||
| PubChem[1][2] | 10197984 | ||
| Hemijski podaci | |||
| Formula | C7H9NO5 | ||
| Mol. masa | 187,150 g/mol | ||
| SMILES | eMolekuli & PubHem | ||
| |||
| Farmakoinformacioni podaci | |||
| Trudnoća | ? | ||
| Pravni status | |||
LY-379,268 je lek koji se koristi u neurološkim istraživanjima. On deluje kao potentan i selektivan agonist za grupu II metabotropnih glutamatnih receptora (mGluR2/3).
On je izveden iz starijeg agonista mGluR groupe II eglumegada,[3] i doveo je do razvoja potentnijeg jedinjenja LY-404,039,[4] ali se još uvek koristi u istraživanjima. LY-379,268 ima sedativne, neuroprotektivne, antizavisničke i antikonvulsantske efekte na životinjama,[5][6][7] i blokira dejstvo PCP-a i DOI-a,[8][9][10][11] što je dovelo do ispitivanja LY-379,268 i sličnih jedinjenja kao potencijalnih antipsihotičnih lekova za tretman šizovrenije.[12][13]
Reference[uredi | uredi kod]
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519.
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Monn JA, Valli MJ, Massey SM, Hansen MM, Kress TJ, Wepsiec JP, Harkness AR, Grutsch JL, Wright RA, Johnson BG, Andis SL, Kingston A, Tomlinson R, Lewis R, Griffey KR, Tizzano JP, Schoepp DD (March 1999). „Synthesis, pharmacological characterization, and molecular modeling of heterobicyclic amino acids related to (+)-2-aminobicyclo[3.1.0] hexane-2,6-dicarboxylic acid (LY354740): identification of two new potent, selective, and systemically active agonists for group II metabotropic glutamate receptors”. Journal of Medicinal Chemistry 42 (6): 1027–40. DOI:10.1021/jm980616n. PMID 10090786.
- ↑ Rorick-Kehn LM, Johnson BG, Burkey JL, Wright RA, Calligaro DO, Marek GJ, Nisenbaum ES, Catlow JT, Kingston AE, Giera DD, Herin MF, Monn JA, McKinzie DL, Schoepp DD (April 2007). „Pharmacological and pharmacokinetic properties of a structurally novel, potent, and selective metabotropic glutamate 2/3 receptor agonist: in vitro characterization of agonist (-)-(1R,4S,5S,6S)-4-amino-2-sulfonylbicyclo[3.1.0]-hexane-4,6-dicarboxylic acid (LY404039)”. The Journal of Pharmacology and Experimental Therapeutics 321 (1): 308–17. DOI:10.1124/jpet.106.110809. PMID 17204749.
- ↑ Cai Z, Xiao F, Fratkin JD, Rhodes PG (December 1999). „Protection of neonatal rat brain from hypoxic-ischemic injury by LY379268, a Group II metabotropic glutamate receptor agonist”. Neuroreport 10 (18): 3927–31. DOI:10.1097/00001756-199912160-00037. PMID 10716235.
- ↑ Moldrich RX, Jeffrey M, Talebi A, Beart PM, Chapman AG, Meldrum BS (July 2001). „Anti-epileptic activity of group II metabotropic glutamate receptor agonists (-)-2-oxa-4-aminobicyclo[3.1.0hexane-4,6-dicarboxylate (LY379268) and (-)-2-thia-4-aminobicyclo[3.1.0]hexane-4,6-dicarboxylate (LY389795)”]. Neuropharmacology 41 (1): 8–18. DOI:10.1016/S0028-3908(01)00044-2. PMID 11445181.
- ↑ Uys JD, LaLumiere RT (November 2008). „Glutamate: the new frontier in pharmacotherapy for cocaine addiction”. CNS & Neurological Disorders Drug Targets 7 (5): 482–91. DOI:10.2174/187152708786927868. PMID 19128205.
- ↑ Cartmell J, Monn JA, Schoepp DD (March 2000). „Attenuation of specific PCP-evoked behaviors by the potent mGlu2/3 receptor agonist, LY379268 and comparison with the atypical antipsychotic, clozapine”. Psychopharmacology 148 (4): 423–9. DOI:10.1007/s002130050072. PMID 10928316.
- ↑ Greenslade RG, Mitchell SN (July 2004). „Selective action of (-)-2-oxa-4-aminobicyclo[3.1.0]hexane-4,6-dicarboxylate (LY379268), a group II metabotropic glutamate receptor agonist, on basal and phencyclidine-induced dopamine release in the nucleus accumbens shell”. Neuropharmacology 47 (1): 1–8. DOI:10.1016/j.neuropharm.2004.02.015. PMID 15165829.
- ↑ Kłodzinska A, Bijak M, Tokarski K, Pilc A (September 2002). „Group II mGlu receptor agonists inhibit behavioural and electrophysiological effects of DOI in mice”. Pharmacology, Biochemistry, and Behavior 73 (2): 327–32. DOI:10.1016/S0091-3057(02)00845-6. PMID 12117586.
- ↑ Molinaro G, Traficante A, Riozzi B, Di Menna L, Curto M, Pallottino S, Nicoletti F, Bruno V, Battaglia G (August 2009). „Activation of mGlu2/3 metabotropic glutamate receptors negatively regulates the stimulation of inositol phospholipid hydrolysis mediated by 5-hydroxytryptamine2A serotonin receptors in the frontal cortex of living mice”. Molecular Pharmacology 76 (2): 379–87. DOI:10.1124/mol.109.056580. PMID 19439499.
- ↑ Carter K, Dickerson J, Schoepp DD, Reilly M, Herring N, Williams J, Sallee FR, Sharp JW, Sharp FR (December 2004). „The mGlu2/3 receptor agonist LY379268 injected into cortex or thalamus decreases neuronal injury in retrosplenial cortex produced by NMDA receptor antagonist MK-801: possible implications for psychosis”. Neuropharmacology 47 (8): 1135–45. DOI:10.1016/j.neuropharm.2004.08.018. PMID 15567423.
- ↑ Imre G (2007). „The preclinical properties of a novel group II metabotropic glutamate receptor agonist LY379268”. CNS Drug Reviews 13 (4): 444–64. DOI:10.1111/j.1527-3458.2007.00024.x. PMID 18078428.