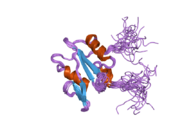RAS p21 proteinski aktivator 1
| edit |
| RAS p21 proteinski aktivator (Protein aktivacije GTPaze) 1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
 PDB rendering based on 1wer. | |||||||||||
| Dostupne strukture | |||||||||||
| 1WER, 1WQ1, 2GQI, 2GSB, 2J05, 2J06, 2M51, 4FSS | |||||||||||
| Identifikatori | |||||||||||
| Simboli | RASA1; CM-AVM; CMAVM; GAP; PKWS; RASA; RASGAP; p120GAP; p120RASGAP | ||||||||||
| Vanjski ID | OMIM: 139150 MGI: 97860 HomoloGene: 2168 GeneCards: RASA1 Gene | ||||||||||
| |||||||||||
| Pregled RNK izražavanja | |||||||||||
 | |||||||||||
 | |||||||||||
| podaci | |||||||||||
| Ortolozi | |||||||||||
| Vrsta | Čovek | Miš | |||||||||
| Entrez | 5921 | 218397 | |||||||||
| Ensembl | ENSG00000145715 | ENSMUSG00000021549 | |||||||||
| UniProt | P20936 | E9PYG6 | |||||||||
| Ref. Sekv. (iRNK) | NM_002890 | NM_145452 | |||||||||
| Ref. Sekv. (protein) | NP_002881 | NP_663427 | |||||||||
| Lokacija (UCSC) | Chr 5: 86.56 - 86.69 Mb | Chr 13: 85.21 - 85.29 Mb | |||||||||
| PubMed pretraga | [1] | [2] | |||||||||
RAS p21 proteinski aktivator 1 ili RasGAP (aktivirajući protein Ras GTPaze), takođe poznat kao RASA1, je 120-kDa citosolni ljudski protein koji ima dve glavne aktivnosti:
- Inakctivacija Ras proteina iz njegove aktivne GTP-vezane forme do njegove neaktivne GDP-vezane forme pojačavanjem endogene GTPazne Ras aktivnosti, preko njegovog C-terminalnog GAP domena
- Mitogena transmisija signala u smeru daljih interakcionih partnera putemn njegovih N-terminalnih SH2-SH3-SH2 domena
Protein kodiran ovim genom je lociran u citoplazmi i deo je GAP1 familije GTPazno-aktiviranih proteina. Ovaj genski produkt stimuliše GTPaznu aktivnost normalnog RAS p21, ali ne i njegovog onkogenog pandana. Delujući kao supresor RAS funkcije, ovaj protein pojačava slabu unutrašnju GTPaznu aktivnost RAS proteina, što dovodi do inaktiviranje GDP-vezane forme RAS-a, čime pospešuje kontrolu ćelijske proliferacije i diferencijacije. Mutacije koje dovode do promena u mestima vezivanja ovog proteina su vezane za bazalne ćelijske kacinome. Alternativnim splajsovanjem se formiraju dve izoforme, pri čemu kraća izoforma, kojoj nedostaje N-terminusni hidrofobni region zadržava aktivnost. Ona je izobilno izražena u materičnim, ali ne i odraslim tkivima.[1]
RasGAP sadrži jedan SH3 domen i dva SH2 domena, PH domen, i GAP domen.
RAS p21 proteinski aktivator 1 formira interakcije sa:
iRNK može da formira interakcije sa Mir-132 mikroRNK. Ovaj protein učestvuje u angiogenezi.[28]
- ↑ „Entrez Gene: RASA1 RAS p21 protein activator (GTPase activating protein) 1”.
- ↑ Chow A, Gawler D (October 1999). „Mapping the site of interaction between annexin VI and the p120GAP C2 domain”. FEBS Lett. 460 (1): 166-72. DOI:10.1016/s0014-5793(99)01336-8. PMID 10571081.
- ↑ Lee H, Park DS, Wang XB, Scherer PE, Schwartz PE, Lisanti MP (September 2002). „Src-induced phosphorylation of caveolin-2 on tyrosine 19. Phospho-caveolin-2 (Tyr(P)19) is localized near focal adhesions, remains associated with lipid rafts/caveolae, but no longer forms a high molecular mass hetero-oligomer with caveolin-1”. J. Biol. Chem. 277 (37): 34556-67. DOI:10.1074/jbc.M204367200. PMID 12091389.
- ↑ Trentin GA, Yin X, Tahir S, Lhotak S, Farhang-Fallah J, Li Y, Rozakis-Adcock M (April 2001). „A mouse homologue of the Drosophila tumor suppressor l(2)tid gene defines a novel Ras GTPase-activating protein (RasGAP)-binding protein”. J. Biol. Chem. 276 (16): 13087-95. DOI:10.1074/jbc.M009267200. PMID 11116152.
- ↑ Dunant NM, Wisniewski D, Strife A, Clarkson B, Resh MD (May 2000). „The phosphatidylinositol polyphosphate 5-phosphatase SHIP1 associates with the dok1 phosphoprotein in bcr-Abl transformed cells”. Cell. Signal. 12 (5): 317-26. DOI:10.1016/s0898-6568(00)00073-5. PMID 10822173.
- ↑ Yamanashi Y, Baltimore D (January 1997). „Identification of the Abl- and rasGAP-associated 62 kDa protein as a docking protein, Dok”. Cell 88 (2): 205-11. DOI:10.1016/s0092-8674(00)81841-3. PMID 9008161.
- ↑ Némorin JG, Duplay P (May 2000). „Evidence that Llck-mediated phosphorylation of p56dok and p62dok may play a role in CD2 signaling”. J. Biol. Chem. 275 (19): 14590-7. DOI:10.1074/jbc.275.19.14590. PMID 10799545.
- ↑ Holland SJ, Gale NW, Gish GD, Roth RA, Songyang Z, Cantley LC, Henkemeyer M, Yancopoulos GD, Pawson T (July 1997). „Juxtamembrane tyrosine residues couple the Eph family receptor EphB2/Nuk to specific SH2 domain proteins in neuronal cells”. EMBO J. 16 (13): 3877-88. DOI:10.1093/emboj/16.13.3877. PMC 1170012. PMID 9233798.
- ↑ Zisch AH, Pazzagli C, Freeman AL, Schneller M, Hadman M, Smith JW, Ruoslahti E, Pasquale EB (January 2000). „Replacing two conserved tyrosines of the EphB2 receptor with glutamic acid prevents binding of SH2 domains without abrogating kinase activity and biological responses”. Oncogene 19 (2): 177-87. DOI:10.1038/sj.onc.1203304. PMID 10644995.
- ↑ Hock B, Böhme B, Karn T, Feller S, Rübsamen-Waigmann H, Strebhardt K (July 1998). „Tyrosine-614, the major autophosphorylation site of the receptor tyrosine kinase HEK2, functions as multi-docking site for SH2-domain mediated interactions”. Oncogene 17 (2): 255-60. DOI:10.1038/sj.onc.1201907. PMID 9674711.
- ↑ Koehler JA, Moran MF (May 2001). „RACK1, a protein kinase C scaffolding protein, interacts with the PH domain of p120GAP”. Biochem. Biophys. Res. Commun. 283 (4): 888-95. DOI:10.1006/bbrc.2001.4889. PMID 11350068.
- ↑ Briggs SD, Bryant SS, Jove R, Sanderson SD, Smithgall TE (June 1995). „The Ras GTPase-activating protein (GAP) is an SH3 domain-binding protein and substrate for the Src-related tyrosine kinase, Hck”. J. Biol. Chem. 270 (24): 14718-24. DOI:10.1074/jbc.270.24.14718. PMID 7782336.
- ↑ 13,0 13,1 Giglione C, Gonfloni S, Parmeggiani A (June 2001). „Differential actions of p60c-Src and Lck kinases on the Ras regulators p120-GAP and GDP/GTP exchange factor CDC25Mm”. Eur. J. Biochem. 268 (11): 3275-83. DOI:10.1046/j.1432-1327.2001.02230.x. PMID 11389730.
- ↑ Molloy DP, Owen D, Grand RJ (July 1995). „Ras binding to a C-terminal region of GAP”. FEBS Lett. 368 (2): 297-303. DOI:10.1016/0014-5793(95)00657-u. PMID 7628625.
- ↑ Sprang SR (July 1997). „GAP into the breach”. Science 277 (5324): 329-30. DOI:10.1126/science.277.5324.329. PMID 9518363.
- ↑ Liu YF, Deth RC, Devys D (March 1997). „SH3 domain-dependent association of huntingtin with epidermal growth factor receptor signaling complexes”. J. Biol. Chem. 272 (13): 8121-4. DOI:10.1074/jbc.272.13.8121. PMID 9079622.
- ↑ Seely BL, Reichart DR, Staubs PA, Jhun BH, Hsu D, Maegawa H, Milarski KL, Saltiel AR, Olefsky JM (August 1995). „Localization of the insulin-like growth factor I receptor binding sites for the SH2 domain proteins p85, Syp, and GTPase activating protein”. J. Biol. Chem. 270 (32): 19151-7. DOI:10.1074/jbc.270.32.19151. PMID 7642582.
- ↑ Sánchez-Margalet V, Najib S (October 2001). „Sam68 is a docking protein linking GAP and PI3K in insulin receptor signaling”. Mol. Cell. Endocrinol. 183 (1-2): 113-21. DOI:10.1016/s0303-7207(01)00587-1. PMID 11604231.
- ↑ Jabado N, Jauliac S, Pallier A, Bernard F, Fischer A, Hivroz C (September 1998). „Sam68 association with p120GAP in CD4+ T cells is dependent on CD4 molecule expression”. J. Immunol. 161 (6): 2798-803. PMID 9743338.
- ↑ Koch CA, Moran MF, Anderson D, Liu XQ, Mbamalu G, Pawson T (March 1992). „Multiple SH2-mediated interactions in v-src-transformed cells”. Mol. Cell. Biol. 12 (3): 1366-74. PMC 369570. PMID 1545818.
- ↑ Ger M, Zitkus Z, Valius M (October 2011). „Adaptor protein Nck1 interacts with p120 Ras GTPase-activating protein and regulates its activity”. Cell. Signal. 23 (10): 1651-8. DOI:10.1016/j.cellsig.2011.05.019. PMID 21664272.
- ↑ Farooqui T, Kelley T, Coggeshall KM, Rampersaud AA, Yates AJ (1999). „GM1 inhibits early signaling events mediated by PDGF receptor in cultured human glioma cells”. Anticancer Res. 19 (6B): 5007-13. PMID 10697503.
- ↑ Ekman S, Kallin A, Engström U, Heldin CH, Rönnstrand L (March 2002). „SHP-2 is involved in heterodimer specific loss of phosphorylation of Tyr771 in the PDGF beta-receptor”. Oncogene 21 (12): 1870-5. DOI:10.1038/sj.onc.1205210. PMID 11896619.
- ↑ Chow A, Davis AJ, Gawler DJ (March 2000). „Identification of a novel protein complex containing annexin VI, Fyn, Pyk2, and the p120(GAP) C2 domain”. FEBS Lett. 469 (1): 88-92. DOI:10.1016/s0014-5793(00)01252-7. PMID 10708762.
- ↑ Zrihan-Licht S, Fu Y, Settleman J, Schinkmann K, Shaw L, Keydar I, Avraham S, Avraham H (March 2000). „RAFTK/Pyk2 tyrosine kinase mediates the association of p190 RhoGAP with RasGAP and is involved in breast cancer cell invasion”. Oncogene 19 (10): 1318-28. DOI:10.1038/sj.onc.1203422. PMID 10713673.
- ↑ Cacalano NA, Sanden D, Johnston JA (May 2001). „Tyrosine-phosphorylated SOCS-3 inhibits STAT activation but binds to p120 RasGAP and activates Ras”. Nat. Cell Biol. 3 (5): 460-5. DOI:10.1038/35074525. PMID 11331873.
- ↑ Brott BK, Decker S, O'Brien MC, Jove R (October 1991). „Molecular features of the viral and cellular Src kinases involved in interactions with the GTPase-activating protein”. Mol. Cell. Biol. 11 (10): 5059-67. PMC 361505. PMID 1717825.
- ↑ Anand S, Majeti BK, Acevedo LM, Murphy EA, Mukthavaram R, Scheppke L, Huang M, Shields DJ, Lindquist JN, Lapinski PE, King PD, Weis SM, Cheresh DA (2010). „MicroRNA-132–mediated loss of p120RasGAP activates the endothelium to facilitate pathological angiogenesis”. Nat Med 16 (8): 909–14. DOI:10.1038/nm.2186. PMC 3094020. PMID 20676106.
- Tocque B, Delumeau I, Parker F, et al. (1997). „Ras-GTPase activating protein (GAP): a putative effector for Ras”. Cell. Signal. 9 (2): 153–8. DOI:10.1016/S0898-6568(96)00135-0. PMID 9113414.
- Boon LM, Mulliken JB, Vikkula M (2005). „RASA1: variable phenotype with capillary and arteriovenous malformations”. Curr. Opin. Genet. Dev. 15 (3): 265–9. DOI:10.1016/j.gde.2005.03.004. PMID 15917201.