Tečno agregatno stanje



Tečnosti ili tekućine su materije tečnog agregatnog stanja.[5] Takve materije nemaju stalan oblik, ali imaju stalnu zapreminu, jer su privlačne sile među njihovim česticama slabije pa se mogu slobodnije kretati. Tečnosti, prema tome, lako menjaju oblik odnosno zauzimaju oblik posude u kojoj se nalaze. U hemiji se materije tečnog agregatnog stanja označavaju malim slovom L (eng. liquid - tečnost). U periodnom sistemu elemenata najmanje je tečnih elemenata,[6] dok su molekularne tečnosti vrlo rasprostranjene u prirodi.
Tečnost je skoro nekompresibilan fluid[7][8] koji zadržava (skoro) konstantnu zapreminu nezavisno od pritiska. Kao takva, tečnost je jedno od četiri fundamentalna stanja materije (pri čemu su druga: čvrsto stanje, gas, i plazma), i ona je jedino stanje sa određenom zapreminom bez fiksnog oblika. Tečnost je sačinjena od malih vibrirajućih čestica materije, kao što su atomi, koji su povezani intermolekularnim vezama.[9][10] Voda je daleko najrasprostranjenija tečnost na Zemlji. Poput gasova, tečnost ima sposobnost proticanja i zauzima oblik suda. Vežina tečnosti je otporna na kompresiju, mada se neke mogu komprimovati. Za razliku od gasova, tečnost se ne širi da bi zauzela svaki deo prostora u sudu, i održava relativno konstantnu gustinu. Distinktno svojstvo tečnog stanja je površinski napon,[3][4] koji dovodi do fenomena vlaženja.[11][12]
Gustina tečnosti je obično blizo gustine čvrste materije, i znatno je veća od gasa. Stoga su tečne i čvrste materije nazvanje kondenzovanim materijama.[13] S druge strane, tečnosti i gasovi imaju zajeničku sposobnost tečenja, i nazivaju se fluidima.[14] Mada je tečna voda izobilna na Zemlji, to stanje materije je zapravo najmanje zastupljeno u poznatom svemiru, pošto je za postojanje tečnosti neophodan relativno uzak opseg temperature/pritiska. Većina poznate materije u svemiru je u gasovitoj formi (sa tragovima detektabilne čvrste materije) kao interstelarni oblaci ili obliku plazme u zvezdama.
Uvod[uredi | uredi kod]
Tečnost je jedno od četiri primarna stanja materija, dok su ostala čvrsto stanje, gas i plazma. Tečnost je fluid. Za razliku od čvrste materije, molekuli tečnosti imaju znatno veći broj stepena slobode kretanja. Sile koje drže molekule tečnosti zajedno u čvrstoj materiji su samo privremene u tečnosti, što omogućava tečnosti da teče, dok čvrste materije ostaju krute.
Tečnost, poput gasa, ispoljava svojstva fluida. Tečnost može da teče, zauzima oblik suda, i ako se stavi u zatvoreni sud ravnomerno raspoređuje primenjeni pritisak na sve površine suda. Ako se tečnost stavi u kesu, njoj se može dati bilo koji željeni oblik. Za razliku od gasa, tečnosti se sa lakoćom uvek ne mešaju sa drugim tečnostima, ne popunjavaju uvek u potpunosti dostupni prostor u sudu, one formiraju svoju sopstvenu površinu, i ne podležu u znatnoj meri komprimovanju, izuzev pod ekstremno visokim pritiscima. Ta svojstva čine tečnost podesnom za primene u hidraulici.[15][16]
Čestice tečnosti su čvrsto vezane, ali one nisu krute. One imaju sposobnost slobodnog kretanja jedna oko druge, što proizvodi ograničene stepene mobilnosti čestica. sa povećanjem temperature, pojačane vibracije molekula uzrokuju povećanje rastojanja između molekula. Kad tečnost dostigne svoju tačku ključanja,[17] kohezivne sile koje blisko vezuju molekule zajedno pucaju, i tečnost prelazi u gasovito stanje (osim slučaja superzagrevanja[18]). Ako se temperatura smanjuje, rastojanje između molekula postaje manje. Kad tečnost dostigne svoju tačku smrzavanja molekuli se obično fiksiraju u veoma specifičnom uređenju,[19] i.e. dolazi do kristalizacije, i veze između njih postaju kruće, čime tečnost prelazi u čvrsto stanje (osim u slučaju superhlađenja[20][21]).
Primeri[uredi | uredi kod]
Jedina dva elementa koja su tečna pod standardnim uslovima temperature i pritiska su: živa i brom. Četiri dodatna elementa imaju tačke topljenja neznatno iznad sobne temperature: francijum, cezijum, galijum i rubidijum.[22] Metalne legure koje su tečne na sobnoj temperaturi su NaK, natrijum-kalijumska metalna legura, galinstan, topljiva tečna legura, i neki amalgami (legure žive).
Čiste supstance koje su tečne pod normalnim uslovima obuhvataju vodu, etanol i mnoge druge organske rastvarače. Tečna voda je od vitalnog značaja u hemiji i biologiji; smatra se da je neophodna za postojanje života.
Neorganske tečnosti obuhvataju vodu, magmu, neorganske nevodene rastvarače i mnoge kiseline.
Među važnim tečnostima u svakodnevnoj upotrebi su tečni rastvori kao što su kućni izbjeljivači, i smeše raznih supstanci kao što su mineralna ulja i benzin, emulzije kao što su prelivi ili majonez, suspenzije poput krvi, i koloidi kao što su boje i mleko.
Mnogi gasovi se mogu prevesti u tečno stanje hlađenjem, čime nastaju tečnosti kao što su tečni kiseonik, tečni azot, tečni vodonik i tečni helijum. Svi gasovi se ne mogu prevesti u tečnost na atmosferskom pritisku, na primer ugljen dioksid se može prevesti u tečnost samo na pritisku iznad 5,1 atm.
Neki materijali se ne mogu klasifikovati u okviru tri klasična stanja materije; oni poseduju svojstva slična čvrstoj materiji i tečnostima. Primeri takivih materijala su tečni kristali, koji se koriste za izradu LCD displeja, i biološke membrane.
Voda[uredi | uredi kod]
Najrasprostanjenija, najpoznatija, najvažnija i najneophodnija tekućina za čovjeka je voda. Ona čini oko 70% površine naše planete a i oko 65% našeg organizma tako da bez nje ne bi bilo ni života...

Primene[uredi | uredi kod]
Tečnosti imaju mnoštvo upotreba, kao lubrikanti, rastvarači, i rashladne tečnosti. U hidrauličkim sistemima, tečnosti se koriste za prenos snage.
U tribologiji, tečnosti se izučavaju zbog njihovih svojstava kao lubrikanti. Lubrikanti kao što su ulja se biraju zbog njihove viskoznosti i protočnih karakteristika koje su podesne širom opsega operacione temperature date komponente. Ulja se često koriste u motorima, transmisijama, obradi metala, i hidrauličkim sistemima zbog njihovig dobrih lubrikacionih svojstava.[23]
Mnoge tečnosti se koriste kao rastvarači, za rastvaranje drugih tečnosti i čvrstih materija. Rastvori nalaze primenu u mnoštvu različitih aplikacija, uključujući boje, zaptivne smese, i lepkove. Nafta i aceton se često koriste u industriji za čišćenje ulja, maziva, i katrana sa delova i mašinerije. Telesni fluidi su rastvori bazirani na vodi.
Surfakanti su obično prisutni u sapunima i deterdžentima. Rastvarači poput alkohola se često koriste kao antimikrobni agensi. Oni nalaze primenu u kozmetici, mastilima, i tečnim obojenim laserima. Oni se koriste u prehrambenoj industriji, u procesim kao što su ekstrakcija biljnog ulja.[24]
Tečnosti obično imaju bolju termalnu provodnost od gasova, i sposobnost tečenja ih čini podesnim za uklanjanje suvišne toplote iz mehaničkih komponenti. Toplota se može ukloniti provođenjem tečnosti kroz toplotne razmenjivačje, kao što su radijatori, ili do uklanjanja toplote može doći putem isparavanja.[25] Vodeni ili glikolni rashlađivači se koriste za sprečavanje pregrevanja motora.[26] Rashlađivači koji se koriste u nuklearnim reaktorima obuhvataju vodu i tečne metale, kao što su natrijum ili bizmut.[27] Tečni propelantni filmovi se koriste za hlađenje potisnih komora raketa.[28] U mašinstvu, se koriste voda i ulje za uklanjanje suvišne toplote, koja može brzo da ošteti obrađivani deo i alat. Tokom perspiracije, znoj uklanja toplotu iz ljudskog tela putem isparavanja. U industriji zagrevanja, ventilacije, i klimatizacije (HVAC), tečnosti kao što je voda se koriste za transfer toplote sa jedne oblasti na drugu.[29]
Tečnost je primarna komponenta hidrauličnih sistema, koji funkcionišu na principu Paskalovog zakona i pružaju snagu tečnosti. Uređaji kao što su pumpe i vodeni točkovi su korišteni za preobražaj kretanja tečnosti u mehanički rad od drevnih vremena. Ulja prolaze kroz hidraulične pumpe, koje transmituju tu silu do hidrauličkih cilindara. Hidraulički uređaji imaju mnoštvo primena, kao što su automobilske kočnice i transmisije, teška oprema, i kontrolni sistemi aviona. Razne hirauličke prese se ekstenzivno koriste za popravku i proizvodnju, za podizanje i presovanje, stezanje i formiranje.[30]
Tečnosti se ponekad koriste u mernim uređajima. Termometri[31] često koriste termalnu ekspanziju tečnosti, kao što je živa, u kombinaciji sa njenom sposobnosti da teče, za indiciranje temperature.[32][33] Manometar koristi težinu tečnosti kao indikator vazdušnog pritiska.[34]
Mehanička svojstva[uredi | uredi kod]
Zapremina[uredi | uredi kod]
Količina tečnosti se obično meri u jedinicama zapremine. One obuhvataju SI jedinicu kubni metar (m3) i njegove delove, a posebno kubni decimeter, koji se najčešće naziva litrom (1 dm3 = 1 L = 0.001 m3), i kubni cantimetar, koji se naziva mililitrom (1 cm3 = 1 mL = 0.001 L = 10−6 m3).
Zapremina date količine tečnosti je fiksna na datoj temperaturi i pritisku. Tečnosti se generalno šire pri zagrevanju, i sakupljaju pri hlađenju. Voda između 0 °C i 4 °C je primetni izuzetak.
Tečnosti imaju malu kompresibilnost. Voda se, na primer, može komprimovati za samo 46,4 dela po milionu za svaku jedinicu povećanja atmosferskog pritiska (bar).[35] Na oko 4000 bara (58.000 psi) pritiska, na sobnoj temperaturi, voda ispoljava samo 11% smanjenja zapremina.[36] Pri izučavanju dinamike fluida, tečnosti se često tretiraju kao nekompresibilne, a to je posebno slučaj u studijama nekompresibilnog protoka. Ta nekompresibilna priroda čini tečnosti podesnim za prenos hidrauličke snage, pošto se veoma malo energije gubi u obliku kompresije.[36] Međutim, neznatna kompresibilnost dovodi do drugih fenomena. Lipanje cevi, zvano vodeni udar, se javlja kad se ventil naglo zatvori, kreirajući ogromno povišenje pritiska na ventilu koje putuje nazad širom sistema. Još jedan fenomenon uzrokovan nestišljivošću tečnosti je kavitacija, pri čemu tečnost u oblasti niskog pritiska isparava i formira mehure, koji zatim kolapsiraju pri ulasku u oblasti visokog pritiska. To uzrokuje popunjavanje praznina na kojima su postojali mehuri tečnošću sa ogromnom, lokalizovanom silom, erodirajući bilo koju susednu čvrstu površinu.[37]
Pritisak i potisak[uredi | uredi kod]
U gravitacionom polju, tečnosti vrše pritisak na zidove suda kao i na predmete u samoj tečnosti. Taj pritisak se prenosi u svim pravcima i povećava se sa dubinom. Ako je tečnost u miru u uniformnom gravitacionom polju, pritisak, p, na bilo kojoj dubini, z, je dat sa
gde:
- označava gustinu tečnosti (pretpostavljenog sadržaja)
- je gravitaciono ubrzanje.
Ova formula pretpostavlja da je pritisak na slobodnoj površini jednak nuli, i da su efekti površinskog napona zanemarljivi.
Objekti uronjeni u tečnosti su podložni fenomenu potiska.[38] (Potisak je primenta u svim fluidima, mada je posebno jak u tečnostima zbog njihove velike gustine.)
Površine[uredi | uredi kod]

Osom ako zapremina tečnosti precizno odgovara zapremini suda, primetna je jedna ili više površina. Površina tečnosti se ponaša kao elastična membrana na kojoj se javlja površinski napon, koji omogućava formiranje kapi i mehura. Površinski talasi, kapilarnih pojava, vlaženje, i kapilarni talasi su druge konsekvence površinskog napona.
Slobodna površina[uredi | uredi kod]
Slobodna površina je površina fluida koja je podložna nultom normalnom stresu i paralelnom smicanju, kao granica između, e.g., tečne vode i vazduha Zemljine atmosfere.
Nivo[uredi | uredi kod]
Nivo tečnosti (npr. nivo vode) je visina asocirana sa slobodnom površinom tečnosti, posebno kad je to najviši deo njene površine. On se može meriti pomoću senzora nivoa.[39]
Protok[uredi | uredi kod]
Viskozitet meri otpor tečnosti koja se deformiše putem smicanja ili ekstenzionalanog stresa.[40]
Kad je tečnost superohlađena do temperature ostakljavanja, viskozitet se dramatično povećava. Tečnost onda postaje viskoelastični medijum, koji pokazuje elastičnost čvrste materije i fluidnost tečnosti, u zavisnosti od vremenske skale opažanja ili frekvencije perturbacije.
Propagacija zvuka[uredi | uredi kod]
Brzina zvuka u tečnosti je data sa , gde K označava modul stišljivosti fluida, a ρ gustinu. Tipična vrednosti za svežu vodu je c=1497 m/s na 25 °C.
Termodinamika[uredi | uredi kod]
Fazni prelazi[uredi | uredi kod]
Na temperaturi ispod tačke ključanja, bilo koja materija u tečnoj formi će isparavati dok kondenzacija gasa iznad nje ne dostigne ravnotežu. U toj tački gas se kondenzuje istom brzinom kojom tečnost isparava. Stoga, tečnost ne može permanentno da postoji, ako se isparena tečnost konstantno uklanja. Tečnost na njenoj tački ključanja će isparavati brže nego što se gas kondenzuje na datom pritisku. Tečnost na ili iznad njene tačke ključanja normalno ključa, mada superzagrejavanje može to da spreči u pojedinim okolnostima.
Na temperaturi ispod tačke smrzavanja, tečnost se kristalizuje, prelazeći u svoje čvrsto stanje. Za razliku od prelaza u gas, ne postoji ravnoteža u ovoj tranziciji pod konstantnim pritiskom, tako da osim ako dođe do superhlađenja, tečnost će konačno potpuno kristalisati. Do toga jedino dolazi pri konstantnom pritisku, tako da na primer voda i led u zatvorenom, jakom sudu mogu da dostignu ravnotežu, gde obe faze postoje. Promena u suprotnom smeru iz čvrste materije u tečnost se naziva topljenje.
Tečnost u svemiru[uredi | uredi kod]
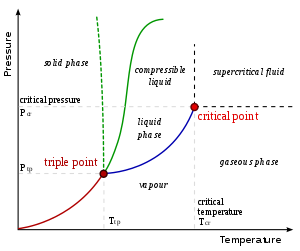
Fazni dijagram objašnajava zašto tečnosti ne postoje u svemiru ili u nekom drugom vakuumu. Pošto je pritisak jednak nuli (izuzev na površinama ili unutrašnjosti planeta ili meseca) voda i druge tečnosti izložene svemiru će odmah proključati ili će se smrznuti u zavisnosti od temperature. U regionima prostora u blizini Zemlje, voda se smrzava ako je Sunce direktno ne obasjava i isparava (sublimira) čim na nju padnu sunčevi zraci. Ako voda postoji kao led na Mesecu, ona može jedino da postoji u zasenčenim rupama gde sunce nikad ne doseže i gde se okolne stene suviše ne zagrevaju. U pojedinim tačkama u blizini orbite Saturna, Sunčeva svetlost je suviše slaba da bi sublimirala led do vodene pare. To je evidentno po dugotrajnosti leda koji sačinjava Saturnove prstenove.
Rastvori[uredi | uredi kod]
Tečnosti mogu da manifestuju nemešljivost. Jedna od poznatih nemešljivih smeša tečnosti iz svakodnevnog života je smeša biljnog ulja i vode u prelivu za salatu. Poznat par tečnosti koje se mešaju su voda i alkohol. Tečne komponente smeše često mogu da budu odvojene jedna od druge putem frakcione destilacije.
Mikroskopska svojstva[uredi | uredi kod]
Statički strukturni faktor[uredi | uredi kod]
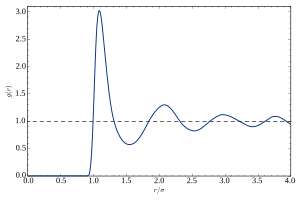
U tečnosti, atomi ne formiraju kristalnu rešetku, niti oni mogu da ispolje bilo koju formu reda na većim rastojanjima. To je evidentno po odsustvu Bragovih pikova u rendgenskoj i neutronskoj difrakciji. Pod normalnim uslovima, difrakcioni patern ima kružnu simetriju, izražavajući izotropiju tečnosti. U radijalnom pravcu, difrakcioni intenzitet glatko osciluje. To se obično opisuje statičkim strukturnim faktorom S(q), sa talasnim brojem q=(4π/λ)sinθ datim talasnom dužinom λ sonde (fotona ili neutrona) i Bragovim uglom θ. Oscilacije S(q) izražavaju bliski red tečnosti, i.e. korelacije između atoma i nekoliko ljuski najbližih, drugih najbližih, ... suseda.
Intuitivniji opis tih korelacija je dat putem funkcije radijalne distribucije g(r), koja je bazično Furijeva transformacija S(q). Ona predstavlja prostorni prosek temporalnog snimka para korelacija u tečnosti.
Disperzija zvuka i strukturna relaksacija[uredi | uredi kod]
Gornji izraz za brzinu zvuka sadrži nemešljivost K. Ako je K nezavisna od frekvencije onda se tečnost ponaša kao linearni medijum, tako da se zvuk širi bez disipacije i bez sprezanja modova. U realnosti, svaka tečnost pokazuje izvesnu disperziju: sa povećanjem frekvencije, K prelazi od niske frekvencije, limita sličnog tečnosti, do visoke frekvencije, limita sličnom čvrstom stanju . U normalnim tečnostima, većina tih prelaza se događa na frekvencijama između GHz i THz, ponekad zvanih hiperzvuk.
Na sub-GHz frekvencijama, normalna tečnosti ne može da održi S talase: limit nulte frekvencije modula smicanja je . To se ponekad smatra definišućim svojstvom tečnosti.[41][42] Međutim, poput modula stišljivosti K, moduo smicanja G je zavistan od frekvencije, i na hiperzvučnim frekvencijama on pokazuje sličan prelaz iz limita sličnog tečnosti u limit sličan čvrstom stanju, ne nultu vrednost .
U skladu sa Kramers-Kronigovom relacijom, disperzija brzine zvuka (predstavljena realnim delom K ili G) ide uz maksimum zvučnog prigušenja (disipacija, predstavljena imaginarnim delom K ili G). Prema teoriji linearnog responsa, Furierova transformacija K ili G opisuje način na koji se sistem vraća u ravnotežu nakon spoljašnje perturbacije; iz tog razloga, disperzioni korak u GHz..THz regionu se isto takon naziva sturkturnom relaksacijom. Prema fluktuaciono-disipacionoj teoremi, relaksacija prema ravnoteži je blisko povezana sa fluktuacijama u ravnoteže. Gustina fluktuacija vezanih za zbučne talase se može eksperimentalno uočiti u vidu Brilouinovog rasipanja.
Pri superhlađenju tečnosti u blizini staklene tranzicije, prelaz iz tečnosti sličnog u čvrstoj materiji sličan respons odgovara prelazu iz GHz u MHz, kHz, Hz, ...; ekvivalentno tome, karakteristično vreme strukturne relaksacije se povećava sa ns na μs, ms, s, ... To je mikroskopsko objašnjenje za gore pomenuto viskoelastično ponašanje tečnosit koje formiraju staklo.
Efekti asocijacije[uredi | uredi kod]
Mehanizmi atomske/molekulske difuzije (ili pomeranja čestica) u čvrstoj materiji su blisko srodni sa mehanizmima viskoznog protoka i solidifikacije tečnih materijala. Opisi viskoziteta u pogledu molekularnog „slobodnog prostora“ u tečnosti[43] su modifikovani po potrebi da bi se uzele u obzir tečnosti za čije molekule je poznato su „asocirani“ u tečnom stanju na običnim temperaturama. Kad se različiti molekuli kombinuju u grupe asociranih molekula, oni formiraju polukrute sisteme u datim prostornim oblastima koje su ranije bile dostupne kao slobodni prostor za mobilne molekule. To dovodi do povećanje viskoziteta nakon hlađenja usled tendencije većine supstanci da budu asocirane nakon hlađenja.[44]
Slični argumenti se koriste za opisivanje dejstva pritiska na viskozitet, pri čemu se može pretpostaviti da je viskozitet prvenstveno funkcija zapremine za tečnosti sa konačnom kompresibilnošću. Očekuje se povećanje viskoziteta sa porastom pritiska. Ako se zapremina povećava toplotom i redukuje pritiskom, viskozitet se ne menja.
Lokalna tendencija da se molekuli orijentišu u malim grupama daje tečnosti (kao što je navedeno ranije) ogređeni stepen asocijacije. Ta asocijacija rezultira u znatnom „unutrašnjem pritisku“ tečnosti, koji je skoro potpuno uzrokovan tim molekulima koji su usled svojih privremeno malih brzina (sledstveno Maksvelovoj distribuciji) grupisani sa drugim molekulima. Unutrašnji pritisak između nekoliko takvih molekula može da odgovara pritisku između grupa molekula u čvrstoj formi.
Reference[uredi | uredi kod]
- ↑ M.A. Wahab (2005). Solid State Physics: Structure and Properties of Materials. Alpha Science. str. 1–3. ISBN 1-84265-218-4.
- ↑ Cutnell, John D.; Kenneth W. Johnson (2006). Essentials of Physics. Wiley Publishing.
- ↑ 3,0 3,1 Roger P. Woodward, Ph.D. (PDF). Surface Tension Measurements Using the Drop Shape Method. First Ten Angstroms. Arhivirano iz originala na datum 2008-12-17. Pristupljeno 2008-11-05.
- ↑ 4,0 4,1 F.K.Hansen; G. Rodsrun (1991). „Surface tension by pendant drop. A fast standard instrument using computer image analysis”. Colloid and Interface Science 141: 1–12. DOI:10.1016/0021-9797(91)90296-K.
- ↑ Peter Atkins, Julio de Paula (2001). Physical Chemistry (7th edition izd.). W. H. Freeman. ISBN 0716735393.
- ↑ Emsley, John (2011). Nature's Building Blocks: An A-Z Guide to the Elements (New izd.). New York, NY: Oxford University Press. ISBN 978-0-19-960563-7.
- ↑ Fine, Rana A.; Millero, F. J. (1973). „Compressibility of water as a function of temperature and pressure”. Journal of Chemical Physics 59 (10): 5529–5536. Bibcode 1973JChPh..59.5529F. DOI:10.1063/1.1679903.
- ↑ Hugh D. Young; Roger A. Freedman. University Physics with Modern Physics. Addison-Wesley; 2012. ISBN 978-0-321-69686-1. p. 356.
- ↑ Donald A. McQuarrie, John D. Simon (1997). Physical Chemistry: A Molecular Approach (1st edition izd.). University Science Books. ISBN 0935702997.
- ↑ Volland, Dr. Walt. „"Intermolecular" Forces”. Pristupljeno 20. 9. 2009.
- ↑ Dezellus, O. and N. Eustathopoulos (2010). "Fundamental issues of reactive wetting by liquid metals." Journal of Materials Science 45(16): 4256-4264.
- ↑ Han Hu, Hai-Feng Ji, and Ying Sun, Phys. Chem. Chem. Phys., 15, (2013) 16557
- ↑ Taylor, Philip L. (2002). A Quantum Approach to Condensed Matter Physics. Cambridge University Press. ISBN 0-521-77103-X.
- ↑ Bird, Byron; Stewart, Warren; Lightfoot, Edward (2007). Transport Phenomena. New York: Wiley, Second Edition. str. 912. ISBN 0-471-41077-2.
- ↑ Horst Beer: 100 Jahre Entwicklung und Einsatz der Hydraulik im Osten Deutschlands. Ein Beitrag zur Technik- und Industriegeschichte. GNN-Verlag, Schkeuditz 2008, ISBN 978-3-89819-240-8.
- ↑ H. Exner, R. Freitag, H. Geis, R. Lang. J. Oppolzer: Der Hydraulik Trainer. Band 1: Hydraulik – Grundlagen und Komponenten. . 3. überarbeitete Auflage. Herausgegeben von Bosch Rexroth AG. Mannesmann Rexroth, Lohr 2002, ISBN 3-933698-30-8.
- ↑ Goldberg, David E. (1988). 3,000 Solved Problems in Chemistry (1st izd.). McGraw-Hill. section 17.43, p. 321. ISBN 0-07-023684-4.
- ↑ Atmosphere-ocean Interaction By Eric Bradshaw Kraus, Joost A. Businger Published by Oxford University Press US, 1994 ISBN 0-19-506618-9, pg 60.
- ↑ Haynes, William M., ur. (2011). CRC Handbook of Chemistry and Physics (92nd ed. izd.). CRC Press. ISBN 1439855110.
- ↑ Debenedetti, P. G.; Stanley, H. E. (2003). „Supercooled and Glassy Water”. Physics Today 56 (6): 40–46. Bibcode 2003PhT....56f..40D. DOI:10.1063/1.1595053.
- ↑ Giovambattista, N.; Angell, C. A.; Sciortino, F.; Stanley, H. E. (July 2004). „Glass-Transition Temperature of Water: A Simulation Study”. Physical Review Letters 93 (4): 047801. arXiv:cond-mat/0403133. Bibcode 2004PhRvL..93d7801G. DOI:10.1103/PhysRevLett.93.047801. PMID 15323794.
- ↑ Theodore Gray, The Elements: A Visual Exploration of Every Known Atom in the Universe New York: Workman Publishing, 2009 p. 127 ISBN 1-57912-814-9
- ↑ Theo Mang, Wilfried Dressel ’’Lubricants and lubrication’’, Wiley-VCH 2007 ISBN 3-527-31497-0
- ↑ George Wypych ’’Handbook of solvents’’ William Andrew Publishing 2001 pp. 847–881 ISBN 1-895198-24-0
- ↑ N. B. Vargaftik ’’Handbook of thermal conductivity of liquids and gases’’ CRC Press 1994 ISBN 0-8493-9345-0
- ↑ Jack Erjavec ’’Automotive technology: a systems approach’’ Delmar Learning 2000 p. 309 ISBN 1-4018-4831-1
- ↑ Gerald Wendt ’’The prospects of nuclear power and technology’’ D. Van Nostrand Company 1957 p. 266
- ↑ ’’Modern engineering for design of liquid-propellant rocket engines’’ by Dieter K. Huzel, David H. Huang – American Institute of Aeronautics and Astronautics 1992 p. 99 ISBN 1-56347-013-6
- ↑ Thomas E Mull ’’HVAC principles and applications manual’’ McGraw-Hill 1997 ISBN 0-07-044451-X
- ↑ R. Keith Mobley Fluid power dynamics Butterworth-Heinemann 2000 p. vii ISBN 0-7506-7174-2
- ↑ Middleton, W.E.K. (1966). A history of the thermometer and its use in meteorology. Baltimore: Johns Hopkins Press. Reprinted ed. 2002, ISBN 0-8018-7153-0.
- ↑ T.D. McGee (1988) Principles and Methods of Temperature Measurement ISBN 0-471-62767-4
- ↑ T.D. McGee (1988) Principles and Methods of Temperature Measurement page 3, ISBN 0-471-62767-4
- ↑ Bela G. Liptak ’’Instrument engineers’ handbook: process control’’ CRC Press 1999 p. 807 ISBN 0-8493-1081-4
- ↑ Compressibility of Liquids
- ↑ 36,0 36,1 Intelligent Energy Field Manufacturing: Interdisciplinary Process Innovations By Wenwu Zhang -- CRC Press 2011 Page 144
- ↑ Fluid Mechanics and Hydraulic Machines by S. C. Gupta -- Dorling-Kindersley 2006 Page 85
- ↑ „Floater clustering in a standing wave: Capillarity effects drive hydrophilic or hydrophobic particles to congregate at specific points on a wave” (PDF). 23 June 2005.
- ↑ Deeter. „Float Level Sensors”. Pristupljeno 2009-05-05.
- ↑ 40,0 40,1 F. White (2003). Fluid Mechanics. McGraw-Hill. str. 4. ISBN 0-07-240217-2.
- ↑ DOI:10.1017/S0305004100017138
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - ↑ DOI:10.1063/1.1750497
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - ↑ D.B. Macleod (1923). „On a relation between the viscosity of a liquid and its coefficient of expansion”. Trans. Farad. Soc. 19: 6. DOI:10.1039/tf9231900006.
- ↑ G.W Stewart (1930). „The Cybotactic (Molecular Group) Condition in Liquids; the Association of Molecules”. Phys. Rev. 35 (7): 726. Bibcode 1930PhRv...35..726S. DOI:10.1103/PhysRev.35.726.
Literatura[uredi | uredi kod]
- J. P. Hansen, I. R. Mcdonald: Theory of simple Liquids. Elsevier Academic Press, 2006, ISBN 978-0-12-370535-8
- M. P. Allen, D.J. Tildesly: Computer Simulation of Liquids. Oxford University Press, 1989, ISBN 0-19-855645-4








