Malonska kiselina
Izgled
| Malonska kiselina | |||
|---|---|---|---|
| |||

| |||
| Identifikacija | |||
| CAS registarski broj | 141-82-2 | ||
| PubChem[1][2] | 867 | ||
| ChemSpider[3] | 844 | ||
| DrugBank | DB02175 | ||
| KEGG[4] | |||
| ChEBI | 30794 | ||
| ChEMBL[5] | CHEMBL7942 | ||
| Jmol-3D slike | Slika 1 | ||
| |||
| |||
| Svojstva | |||
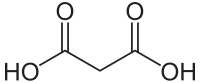
| Molekulska formula | C3H4O4 | ||
| Molarna masa | 104.06 g mol−1 | ||
| Tačka topljenja |
135 | ||
|
Ukoliko nije drugačije napomenuto, podaci se odnose na standardno stanje (25 °C, 100 kPa) materijala | |||
| Infobox references | |||
Malonska kiselina je organsko jedinjenje, koje sadrži 3 atoma ugljenika i ima molekulsku masu od 104,062 Da.[6][7]
| Osobina | Vrednost |
|---|---|
| Broj akceptora vodonika | 4 |
| Broj donora vodonika | 2 |
| Broj rotacionih veza | 2 |
| Particioni koeficijent[8] (ALogP) | -0,4 |
| Rastvorljivost[9] (logS, log(mol/L)) | -0,2 |
| Polarna površina[10] (PSA, Å2) | 74,6 |
Kada su povišene razine malonske kiseline praćene sa povišenim razinama metilmalonske kiseline, to može upućivati na često neprepoznatu[11] metaboličku bolest kombiniranu malonsku i metilmalonsku aciduriju (CMAMMA). Računanjem omjera malonske kiseline i metilmalonske kiseline u krvnoj plazmi može se razlikovati CMAMMA od klasične metilmalonske acidemije.[12]
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519.
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining”. J Cheminform 2 (1): 3. DOI:10.1186/1758-2946-2-3. PMID 20331846.
- ↑ Joanne Wixon, Douglas Kell (2000). „Website Review: The Kyoto Encyclopedia of Genes and Genomes — KEGG”. Yeast 17 (1): 48–55. DOI:10.1002/(SICI)1097-0061(200004)17:1<48::AID-YEA2>3.0.CO;2-H.
- ↑ Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B, Overington JP. (2012). „ChEMBL: a large-scale bioactivity database for drug discovery”. Nucleic Acids Res 40 (Database issue): D1100-7. DOI:10.1093/nar/gkr777. PMID 21948594.
- ↑ Knox C, Law V, Jewison T, Liu P, Ly S, Frolkis A, Pon A, Banco K, Mak C, Neveu V, Djoumbou Y, Eisner R, Guo AC, Wishart DS (2011). „DrugBank 3.0: a comprehensive resource for omics research on drugs”. Nucleic Acids Res. 39 (Database issue): D1035-41. DOI:10.1093/nar/gkq1126. PMC 3013709. PMID 21059682.
- ↑ David S. Wishart, Craig Knox, An Chi Guo, Dean Cheng, Savita Shrivastava, Dan Tzur, Bijaya Gautam, and Murtaza Hassanali (2008). „DrugBank: a knowledgebase for drugs, drug actions and drug targets”. Nucleic Acids Res 36 (Database issue): D901-6. DOI:10.1093/nar/gkm958. PMC 2238889. PMID 18048412.
- ↑ Ghose, A.K., Viswanadhan V.N., and Wendoloski, J.J. (1998). „Prediction of Hydrophobic (Lipophilic) Properties of Small Organic Molecules Using Fragment Methods: An Analysis of AlogP and CLogP Methods”. J. Phys. Chem. A 102: 3762-3772. DOI:10.1021/jp980230o.
- ↑ Tetko IV, Tanchuk VY, Kasheva TN, Villa AE. (2001). „Estimation of Aqueous Solubility of Chemical Compounds Using E-State Indices”. Chem Inf. Comput. Sci. 41: 1488-1493. DOI:10.1021/ci000392t. PMID 11749573.
- ↑ Ertl P., Rohde B., Selzer P. (2000). „Fast calculation of molecular polar surface area as a sum of fragment based contributions and its application to the prediction of drug transport properties”. J. Med. Chem. 43: 3714-3717. DOI:10.1021/jm000942e. PMID 11020286.
- ↑ NIH Intramural Sequencing Center Group; Sloan, Jennifer L; Johnston, Jennifer J; Manoli, Irini; Chandler, Randy J; Krause, Caitlin; Carrillo-Carrasco, Nuria; Chandrasekaran, Suma D i dr.. (2011-09). „Exome sequencing identifies ACSF3 as a cause of combined malonic and methylmalonic aciduria” (en). Nature Genetics 43 (9): 883–886. DOI:10.1038/ng.908. ISSN 1061-4036. PMC PMC3163731. PMID 21841779.
- ↑ de Sain-van der Velden, Monique G. M.; van der Ham, Maria; Jans, Judith J.; Visser, Gepke; Prinsen, Hubertus C. M. T.; Verhoeven-Duif, Nanda M.; van Gassen, Koen L. I.; van Hasselt, Peter M. (2016-02-27). „A New Approach for Fast Metabolic Diagnostics in CMAMMA”. JIMD Reports 30: 15–22. DOI:10.1007/8904_2016_531. ISSN 2192-8304. PMC 5110436. PMID 26915364.
- Clayden Jonathan, Nick Greeves, Stuart Warren, Peter Wothers (2001). Organic chemistry. Oxford, Oxfordshire: Oxford University Press. ISBN 0-19-850346-6.
- Smith, Michael B.; March, Jerry (2007). Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th izd.). New York: Wiley-Interscience. ISBN 0-471-72091-7.
- Katritzky A.R., Pozharskii A.F. (2000). Handbook of Heterocyclic Chemistry. Academic Press. ISBN 0080429882.

