Labetalol
| (IUPAC) ime | |
|---|---|
| (RS)-2-hidroksi-51-hidroksi-2-[(1-metil-3-fenolpropil)amino]etil | |
| Klinički podaci | |
| Identifikatori | |
| ATC kod | ? |
| Hemijski podaci | |
| Formula | ? |
| Farmakoinformacioni podaci | |
| Trudnoća | ? |
| Pravni status | |
benzamid
| image = (+-)-Labetalol Structural Formula V1.svg | width = 250 | image2 = | width2 = | imagename = 1 : 1 racemska smeša | drug_name = Labetalol
| tradename = Trandat | Drugs.com = Monografija | MedlinePlus = a685034 | pregnancy_category = C | legal_status = Rx-only | routes_of_administration = oralno iv
| bioavailability = 25% | protein_bound = 50% | metabolism = hepatički | elimination_half-life = Tableta: 6-8 sati; IV: 5.5 sati | excretion = urin
| CASNo_Ref = ![]() | CAS_number_Ref =
| CAS_number_Ref = ![]() | CAS_number = 36894-69-6
| ATC_prefix = C07
| ATC_suffix = AG01
| PubChem = 3869
| IUPHAR_ligand =
| DrugBank_Ref = Šablon:Drugbankcite
| CAS_number = 36894-69-6
| ATC_prefix = C07
| ATC_suffix = AG01
| PubChem = 3869
| IUPHAR_ligand =
| DrugBank_Ref = Šablon:Drugbankcite
| DrugBank = DB00598
| ChemSpiderID_Ref = ![]() | ChemSpiderID = 3734
| UNII_Ref =
| ChemSpiderID = 3734
| UNII_Ref = ![]() | UNII = R5H8897N95
| KEGG_Ref =
| UNII = R5H8897N95
| KEGG_Ref = ![]() | KEGG = D08106
| ChEBI_Ref =
| KEGG = D08106
| ChEBI_Ref = ![]() | ChEBI = 6343
| ChEMBL_Ref =
| ChEBI = 6343
| ChEMBL_Ref = ![]() | ChEMBL = 429
| ChEMBL = 429
| C=19 | H=24 | N=2 | O=3
| molecular_weight = 328,406 g/mol
| smiles = O=C(c1cc(ccc1O)C(O)CNC(C)CCc2ccccc2)N
| InChI = 1/C19H24N2O3/c1-13(7-8-14-5-3-2-4-6-14)21-12-18(23)15-9-10-17(22)16(11-15)19(20)24/h2-6,9-11,13,18,21-23H,7-8,12H2,1H3,(H2,20,24)
| InChIKey = SGUAFYQXFOLMHL-UHFFFAOYAT
| StdInChI_Ref = ![]() | StdInChI = 1S/C19H24N2O3/c1-13(7-8-14-5-3-2-4-6-14)21-12-18(23)15-9-10-17(22)16(11-15)19(20)24/h2-6,9-11,13,18,21-23H,7-8,12H2,1H3,(H2,20,24)
| StdInChIKey_Ref =
| StdInChI = 1S/C19H24N2O3/c1-13(7-8-14-5-3-2-4-6-14)21-12-18(23)15-9-10-17(22)16(11-15)19(20)24/h2-6,9-11,13,18,21-23H,7-8,12H2,1H3,(H2,20,24)
| StdInChIKey_Ref = ![]() | StdInChIKey = SGUAFYQXFOLMHL-UHFFFAOYSA-N
}}
Labetalol (Normodin, Trandat) je mešoviti alfa/beta adrenergički antagonist, koji se koristi za tretiranje visokog krvnog pritiska.[1]
| StdInChIKey = SGUAFYQXFOLMHL-UHFFFAOYSA-N
}}
Labetalol (Normodin, Trandat) je mešoviti alfa/beta adrenergički antagonist, koji se koristi za tretiranje visokog krvnog pritiska.[1]
Indikcije[uredi | uredi kod]
Koristi se kao tretman trudnoćom indukovanu hipertenziju koja je često vezana za preeklampsiju.[2] On se takođe koristi za tretiranje hronične i akutne hipertenzije feohromocitoma i hipertenzivne krize.[3][4]
Stereokemija[uredi | uredi kod]
Labetalol sadrži dva stereogena centra i sastoji se od četiri stereoizomera. Ovo je mješavina ("R", "R") -, ("S", "R") -, ("R", "S" ) - i ( S , S ) - oblik:
| Stereoizomeri Labetalola | |
|---|---|
 CAS-Nummer: 75659-07-3 |
 CAS-Nummer: 83167-24-2 |
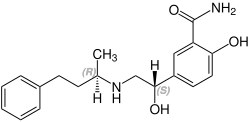 CAS-Nummer: 83167-32-2 |
 CAS-Nummer: 83167-31-1 |
Reference[uredi | uredi kod]
- ↑ Fahed S, Grum DF, Papadimos TJ (2008). „Labetalol infusion for refractory hypertension causing severe hypotension and bradycardia: an issue of patient safety”. Patient Saf Surg 2: 13. DOI:10.1186/1754-9493-2-13. PMC 2429901. PMID 18505576.
- ↑ C. J. Pickles, E. M. Symonds, F. Broughton Pipkin (January 1989). „The fetal outcome in a randomized double-blind controlled trial of labetalol versus placebo in pregnancy-induced hypertension”. BJOG: An International Journal of Obstetrics & Gynaecology 96 (1): 38–43. DOI:10.1111/j.1471-0528.1989.tb01574.x.
- ↑ Katzung, Bertram G. (2006). Basic and clinical pharmacology. New York: McGraw-Hill Medical. str. 170. ISBN 0-07-145153-6.
- ↑ D A Richards, J Tuckman, and B N Prichard (October 1976). „Assessment of alpha- and beta-adrenoceptor blocking actions of labetalol”. Br J Clin Pharmacol 3 (5): 849–855. PMC 1428931. PMID 9968.