Tkivna transglutaminaza
| edit |
| Transglutaminaza 2 (C polipeptid, protein-glutamin-gama-glutamiltransferaza) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
 Prikaz tkivne transglutaminaze baziran na PDB 1KV3. | |||||||||||
| Dostupne strukture | |||||||||||
| 1KV3, 2Q3Z, 3LY6, 3S3J, 3S3P, 3S3S | |||||||||||
| Identifikatori | |||||||||||
| Simboli | TGM2; G-ALPHA-h; GNAH; TG2; TGC | ||||||||||
| Vanjski ID | OMIM: 190196 MGI: 98731 HomoloGene: 3391 GeneCards: TGM2 Gene | ||||||||||
| EC broj | 2.3.2.13 | ||||||||||
| |||||||||||
| Pregled RNK izražavanja | |||||||||||
 | |||||||||||
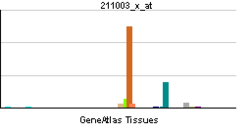 | |||||||||||
 | |||||||||||
| podaci | |||||||||||
| Ortolozi | |||||||||||
| Vrsta | Čovek | Miš | |||||||||
| Entrez | 7052 | 21817 | |||||||||
| Ensembl | ENSG00000198959 | ENSMUSG00000037820 | |||||||||
| UniProt | P21980 | P21981 | |||||||||
| RefSeq (mRNA) | NM_004613 | NM_009373 | |||||||||
| RefSeq (protein) | NP_004604 | NP_033399 | |||||||||
| Lokacija (UCSC) | Chr 20: 36.76 - 36.79 Mb | Chr 2: 158.12 - 158.15 Mb | |||||||||
| PubMed pretraga | [1] | [2] | |||||||||
| Protein-glutamine gamma-glutamyltransferase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifikatori | |||||||||
| EC broj | 2.3.2.13 | ||||||||
| CAS broj | 80146-85-6 | ||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB | RCSB PDB PDBe PDBj PDBsum | ||||||||
| Ontologija gena | AmiGO / EGO | ||||||||
| |||||||||
Tkivna transglutaminaza (tTG, TG2) je 78 kDa, od kalcijuma zavisni enzim (EC 2.3.2.13) iz familije γ-glutamiltransferaza (transglutaminaza).[1][2] Poput drugih transglutaminaza, on formira unakrsne veze u proteinima između ε-amino grupe lizinskih ostataka i γ-karboksamidne grupe glutaminskih ostataka. Nastale inter- ili intramolekulske veze su veoma otporne na proteolizu (proteinsku degradaciju). Pored unakrsnog vezivanja, tTG katalizuje i druge tipove reakcija uključujući deaminaciju, GTP-vezivanje/hidrolizu, i ima izopeptidazno dejstvo.[3] Za razliku od drugih članova transglutaminazne familije, tTG se može naći u intracelularnim i ekstracelularnim prostorima raznih tipova tkiva i prisutan je u mnoštvu različitih organa uključujući srce, jetru, i tanka creva. Intracelularni tTG je izobilan u citosolu, a u malim količinama je prisutan i u jedru i mitohondrijama.[2] Smatra se da intracelularni tTG ima važnu ulogu u apoptozi.[4] U ekstracelularnom prostoru, tTG se vezuje za proteine ekstracelularnog matriksa (ECM),[5] sa posebno jakim vezivanjem za fibronektin.[6] Ekstracelularni tTG uzima udela u ćelijskoh adheziji, ECM stabilizaciji, zarastanju rana, receptorskog signalizaciji, ćelijskoj proliferaciji, i ćelijskoj motilnosti.[2]
tTG je posebno poznat po tome što je autoantigen u celijačnoj bolesti, doživotnoj bolesti usled koje konzumiranje dijetarnog gluten uzrokuje patološki imunski respons koji dovodi do inflamacije tankih creva i naknadne atrofije.[7][8][9]
Reference[uredi | uredi kod]
- ↑ Greška u referenci: Nevaljana oznaka
<ref>; nije zadan tekst za reference po imenuKiraly - ↑ 2,0 2,1 2,2 Greška u referenci: Nevaljana oznaka
<ref>; nije zadan tekst za reference po imenuKlock - ↑ Greška u referenci: Nevaljana oznaka
<ref>; nije zadan tekst za reference po imenuFacchiano - ↑ Greška u referenci: Nevaljana oznaka
<ref>; nije zadan tekst za reference po imenuMcConkey - ↑ Greška u referenci: Nevaljana oznaka
<ref>; nije zadan tekst za reference po imenuLortat-Jacob - ↑ Greška u referenci: Nevaljana oznaka
<ref>; nije zadan tekst za reference po imenuAkimov - ↑ Griffin M, Casadio R, Bergamini CM (December 2002). „Transglutaminases: nature's biological glues”. Biochem. J. 368 (Pt 2): 377–96. DOI:10.1042/BJ20021234. PMC 1223021. PMID 12366374.
- ↑ Greška u referenci: Nevaljana oznaka
<ref>; nije zadan tekst za reference po imenuDiRaimondo - ↑ Greška u referenci: Nevaljana oznaka
<ref>; nije zadan tekst za reference po imenuSabatino
Literatura[uredi | uredi kod]
- Nicholas C. Price, Lewis Stevens (1999). Fundamentals of Enzymology: The Cell and Molecular Biology of Catalytic Proteins (Third izd.). USA: Oxford University Press. ISBN 019850229X.
- Eric J. Toone (2006). Advances in Enzymology and Related Areas of Molecular Biology, Protein Evolution (Volume 75 izd.). Wiley-Interscience. ISBN 0471205036.
- Branden C, Tooze J.. Introduction to Protein Structure. New York, NY: Garland Publishing. ISBN: 0-8153-2305-0.
- Irwin H. Segel. Enzyme Kinetics: Behavior and Analysis of Rapid Equilibrium and Steady-State Enzyme Systems (Book 44 izd.). Wiley Classics Library. ISBN 0471303097.
- Robert A. Copeland (2013). Evaluation of Enzyme Inhibitors in Drug Discovery: A Guide for Medicinal Chemists and Pharmacologists (2nd izd.). Wiley-Interscience. ISBN 111848813X.
- Gerhard Michal, Dietmar Schomburg (2012). Biochemical Pathways: An Atlas of Biochemistry and Molecular Biology (2nd izd.). Wiley. ISBN 0470146842.
refs = [1]
}}
Spoljašnje veze[uredi | uredi kod]
- Endomysial antibodies Arhivirano 2021-05-12 na Wayback Machine-u
- A collection of substrates and interaction partners of TG2 is accessible in the TRANSDAB, an interactive transglutaminase substrate database.
- ↑ Király R, Demény M, Fésüs L (December 2011). „Protein transamidation by transglutaminase 2 in cells: a disputed Ca2+-dependent action of a multifunctional protein”. FEBS J. 278 (24): 4717–39. DOI:10.1111/j.1742-4658.2011.08345.x. PMID 21902809.
- ↑ Klöck C, Diraimondo TR, Khosla C (July 2012). „Role of transglutaminase 2 in celiac disease pathogenesis”. Semin Immunopathol 34 (4): 513–22. DOI:10.1007/s00281-012-0305-0. PMID 22437759.
- ↑ Facchiano F, Facchiano A, Facchiano AM (2006). „The role of transglutaminase-2 and its substrates in human diseases”. Front. Biosci. 11: 1758–73. DOI:10.2741/1921. PMID 16368554.
- ↑ McConkey DJ, Orrenius S (October 1997). „The role of calcium in the regulation of apoptosis”. Biochem. Biophys. Res. Commun. 239 (2): 357–66. DOI:10.1006/bbrc.1997.7409. PMID 9344835.
- ↑ Akimov SS, Krylov D, Fleischman LF, Belkin AM (February 2000). „Tissue transglutaminase is an integrin-binding adhesion coreceptor for fibronectin”. J. Cell Biol. 148 (4): 825–38. DOI:10.1083/jcb.148.4.825. PMC 2169362. PMID 10684262.
- ↑ Diraimondo TR, Klöck C, Khosla C (April 2012). „Interferon-γ activates transglutaminase 2 via a phosphatidylinositol-3-kinase-dependent pathway: implications for celiac sprue therapy”. J. Pharmacol. Exp. Ther. 341 (1): 104–14. DOI:10.1124/jpet.111.187385. PMC 3310700. PMID 22228808.
- ↑ Lortat-Jacob H, Burhan I, Scarpellini A, Thomas A, Imberty A, Vivès RR, Johnson T, Gutierrez A, Verderio EA (May 2012). „Transglutaminase-2 interaction with heparin: identification of a heparin binding site that regulates cell adhesion to fibronectin-transglutaminase-2 matrix”. J. Biol. Chem. 287 (22): 18005–17. DOI:10.1074/jbc.M111.337089. PMC 3365763. PMID 22442151.
- ↑ Di Sabatino A, Vanoli A, Giuffrida P, Luinetti O, Solcia E, Corazza GR (August 2012). „The function of tissue transglutaminase in celiac disease”. Autoimmun Rev 11 (10): 746–53. DOI:10.1016/j.autrev.2012.01.007. PMID 22326684.
