Kinurenin
Prijeđi na navigaciju
Prijeđi na pretragu
| Kinurenin | |||
|---|---|---|---|
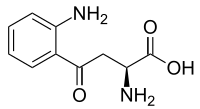
| |||
| IUPAC ime |
| ||
| Drugi nazivi | (S)-Kinurenin | ||
| Identifikacija | |||
| CAS registarski broj | 343-65-7, (D/L) 2922-83-0 (L) 13441-51-5 (D) | ||
| PubChem[1][2] | 846 | ||
| MeSH | |||
| Jmol-3D slike | Slika 1 | ||
| |||
| |||
| Svojstva | |||
| Molekulska formula | C10H12N2O3 | ||
| Molarna masa | 208.21 g mol−1 | ||
|
| |||
| Infobox references | |||
L-Kinurenin je metabolit aminokiseline L-triptofan[3] koji se koristi u produkciji niacina. Smatra se da posreduje pojavu tikova.[4][5]
Kinureninaza katabolizuje konverziju kinurenina u antranilnu kiselinu[6], dok kinurenin—oksoglutarat transaminaza katabolizuje njegovu konverziju u kinurensku kiselinu. Kinurenin 3-hidroksilaza konvertuje kinurenin do 3-hidroksikinurenina.[7]
Literatura[uredi | uredi kod]
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519.
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Moroni F (June 1999). „Tryptophan metabolism and brain function: focus on kynurenine and other indole metabolites”. European Journal of Pharmacology 375 (1-3): 87–100. PMID 10443567.
- ↑ Hoekstra PJ, Anderson GM, Troost PW, Kallenberg CG, Minderaa RB (June 2007). „Plasma kynurenine and related measures in tic disorder patients”. European Child & Adolescent Psychiatry 16 Suppl 1: 71–7. DOI:10.1007/s00787-007-1009-1. PMID 17665285. Pristupljeno 2011-04-06.
- ↑ „Kynurenine potentiates the DOI head shake in mice | DeepDyve - Research. Rent. Read.”. Arhivirano iz originala na datum 2011-07-21. Pristupljeno 06. 04. 2011.
- ↑ European Bioinformatics Institute. „Kynureninase,”.
- ↑ Saito, Y., Hayaishi, O., Rothberg, S. (1957). „Studies on oxygenases; enzymatic formation of 3-hydroxy-L-kynurenine from L-kynurenine”. J. Biol. Chem. 229 (2): 921–34. PMID 13502353.