Bikarbonat
Prijeđi na navigaciju
Prijeđi na pretragu
| Bikarbonat | |||
|---|---|---|---|
| |||

| |||
| Naziv po klasifikaciji | Hidroksidodioksidokarbonat(1−)[1] | ||
| Drugi nazivi | Hidrogenkarbonat[1] | ||
| Identifikacija | |||
| CAS registarski broj | 71-52-3 | ||
| PubChem[2][3] | 769 | ||
| ChemSpider[4] | 749 | ||
| KEGG[5] | |||
| ChEBI | 17544 | ||
| ChEMBL[6] | CHEMBL363707 | ||
| Bajlštajn | 3903504 | ||
| Gmelin Referenca | 49249 | ||
| 3DMet | B00080 | ||
| Jmol-3D slike | Slika 1 Slika 2 | ||
| |||
| |||
| Svojstva | |||
| Molekulska formula | HCO3- | ||
| Molarna masa | 61,0168 g mol−1 | ||
| log P | −0,82 | ||
| pKa | 10,3 | ||
|
| |||
| Infobox references | |||
Bikarbonat (po IUPAC-u hidrogenkarbonat[7]) u neorganskoj hemiji je prelazni oblik u deprotonaciji ugljene kiseline.
Hemijske osobine[uredi | uredi kod]
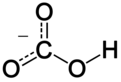
Bikarbonatni jon je anjon sa empirijskom formulom HCO3− i molekularnom masom od 61,01 daltona. Sastoji se od centralnog atoma ugljenika okruženog sa tri atoma kiseonika u trigonalno planarnom rasporedu, i sa atomom vodonika vezanim za jedan od atoma kiseonika.
Jedinjenja bikarbonata[uredi | uredi kod]
Reference[uredi | uredi kod]
- ↑ 1,0 1,1 „hydrogencarbonate (CHEBI:17544)”. Chemical Entities of Biological Interest (ChEBI). UK: European Institute of Bioinformatics. IUPAC Names.
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519.
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining”. J Cheminform 2 (1): 3. DOI:10.1186/1758-2946-2-3. PMID 20331846.
- ↑ Joanne Wixon, Douglas Kell (2000). „Website Review: The Kyoto Encyclopedia of Genes and Genomes — KEGG”. Yeast 17 (1): 48–55. DOI:10.1002/(SICI)1097-0061(200004)17:1<48::AID-YEA2>3.0.CO;2-H.
- ↑ Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B, Overington JP. (2012). „ChEMBL: a large-scale bioactivity database for drug discovery”. Nucleic Acids Res 40 (Database issue): D1100-7. DOI:10.1093/nar/gkr777. PMID 21948594.
- ↑ Nomenclature of Inorganic Chemistry IUPAC Recommendations 2005, IUPAC, p. 137
