Suksametonijum hlorid
Prijeđi na navigaciju
Prijeđi na pretragu
| |||
| |||
| (IUPAC) ime | |||
|---|---|---|---|
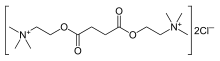
| trimetil[2-({4-okso-4-[2-(trimetilazanijumil)etoksi]butanoil}oksi)etil]azanijum | |||
| Klinički podaci | |||
| Robne marke | Anectine, Quelicin, Sucostrin | ||
| AHFS/Drugs.com | chloride.html Monografija | ||
| Identifikatori | |||
| CAS broj | 306-40-1 | ||
| ATC kod | M03AB01 | ||
| PubChem[1][2] | 5314 | ||
| DrugBank | DB00202 | ||
| ChemSpider[3] | 5123 | ||
| KEGG[4] | C07546 | ||
| ChEMBL[5] | CHEMBL45652 | ||
| Hemijski podaci | |||
| Formula | C14H30N2O4 | ||
| Mol. masa | 290.399 | ||
| SMILES | eMolekuli & PubHem | ||
| |||
| Farmakokinetički podaci | |||
| Izlučivanje | 10% urinom | ||
| Farmakoinformacioni podaci | |||
| Trudnoća | ? | ||
| Pravni status | |||
| Način primene | Intravenozno | ||
Suksametonijum hlorid je kvaternarni relaksant skeletalnih mišića koji se koristi u obliku bromida, hlorida, ili jodida. On je depolarizacioni relaksant, koji deluje nakon oko 30 sekundi i čije dejstvo traje u proseku tri do pet minuta.[6][7][8]
Reference[uredi | uredi kod]
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519.
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining”. J Cheminform 2 (1): 3. DOI:10.1186/1758-2946-2-3. PMID 20331846.
- ↑ Joanne Wixon, Douglas Kell (2000). „Website Review: The Kyoto Encyclopedia of Genes and Genomes — KEGG”. Yeast 17 (1): 48–55. DOI:10.1002/(SICI)1097-0061(200004)17:1<48::AID-YEA2>3.0.CO;2-H.
- ↑ Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B, Overington JP. (2012). „ChEMBL: a large-scale bioactivity database for drug discovery”. Nucleic Acids Res 40 (Database issue): D1100-7. DOI:10.1093/nar/gkr777. PMID 21948594.
- ↑ Jonsson M, Dabrowski M, Gurley DA, Larsson O, Johnson EC, Fredholm BB, Eriksson LI: Activation and inhibition of human muscular and neuronal nicotinic acetylcholine receptors by succinylcholine. Anesthesiology. 2006 Apr;104(4):724-33. PMID 16571968
- ↑ Knox C, Law V, Jewison T, Liu P, Ly S, Frolkis A, Pon A, Banco K, Mak C, Neveu V, Djoumbou Y, Eisner R, Guo AC, Wishart DS (2011). „DrugBank 3.0: a comprehensive resource for omics research on drugs”. Nucleic Acids Res. 39 (Database issue): D1035-41. PMID 21059682.
- ↑ Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, Tzur D, Gautam B, Hassanali M (2008). „DrugBank: a knowledgebase for drugs, drug actions and drug targets”. Nucleic Acids Res 36 (Database issue): D901-6. PMID 18048412.
