Bimatoprost
Prijeđi na navigaciju
Prijeđi na pretragu
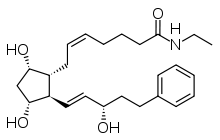
| |||
| (IUPAC) ime | |||
|---|---|---|---|
| 7-[3,5-dihidroksi-2- (3-hidroksi-5-fenil-pent-1-enil)- ciklopentil]-N-etil-hept-5-enamid | |||
| Klinički podaci | |||
| Robne marke | Lumigan | ||
| AHFS/Drugs.com | Monografija | ||
| MedlinePlus | a602030 | ||
| Identifikatori | |||
| CAS broj | 155206-00-1 | ||
| ATC kod | S01EE03 | ||
| PubChem[1][2] | 5311027 | ||
| DrugBank | DB00905 | ||
| ChemSpider[3] | 4470565 | ||
| UNII | QXS94885MZ | ||
| ChEBI | CHEBI:51230 | ||
| ChEMBL[4] | CHEMBL1200963 | ||
| Hemijski podaci | |||
| Formula | C25H37NO4 | ||
| Mol. masa | 415,566 g/mol | ||
| SMILES | eMolekuli & PubHem | ||
| |||
| Farmakoinformacioni podaci | |||
| Licenca | |||
| Trudnoća | C(US) | ||
| Pravni status | ℞-only (SAD) | ||
| Način primene | Topikalni (kapi za oči) | ||
Bimatoprost (Lumigan) je analog prostaglandina. On je prolek koji se koristi topikalni (u obliku kapi za oči) za kontrolu progresa glaukoma i okularne hipertenzije. On redukuje intraokularni pristisak (IOP) putem povećanja odliva fluida iz očiju.[5] Poznato je da se koristi za produžavanje trepavica.[6] FDA je odobrila ovaj način primene decembra 2008. Kozmetička formulacija bimatoprosta je u prodaji pod imenom Latis.[7] Tokom perioda od 2008 - 2011, utvrđeno je da bimatoprost ima sposobnost redukovanja masnog tkiva.[8][9][10]
Reference[uredi | uredi kod]
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519.
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining”. J Cheminform 2 (1): 3. DOI:10.1186/1758-2946-2-3. PMID 20331846.
- ↑ Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B, Overington JP. (2012). „ChEMBL: a large-scale bioactivity database for drug discovery”. Nucleic Acids Res 40 (Database issue): D1100-7. DOI:10.1093/nar/gkr777. PMID 21948594.
- ↑ „Bimatoprost Ophthalmic”. MedlinePlus. 1. 1. 2003.. Arhivirano iz originala na datum 2007-10-05. Pristupljeno 19. 11. 2007.
- ↑ Rundle, Rhonda L. (19. 11. 2007.). „Drug That Lengthens Eyelashes Sets Off Flutter”. The Wall Street Journal. Pristupljeno 19. 11. 2007.
- ↑ „Allergan gets FDA approval for eyelash treatment”. BusinessWeek. Associated Press. 26. 12. 2008.. Pristupljeno 26. 12. 2008.
- ↑ Park J, Cho HK, Moon JI (2011). „Changes to upper eyelid orbital fat from use of topical bimatoprost, travoprost, and latanoprost.”. Japanese Ophthalmological Society 55 (1): 22–27. DOI:10.1007/s10384-010-0904-z. PMID 21331688.
- ↑ Jayaprakasam A, Ghazi-Nouri S. (2010). „Periorbital fat atrophy - an unfamiliar side effect of prostaglandin analogues.”. Orbit 29 (6): 357–359. DOI:10.3109/01676830.2010.527028. PMID 21158579.
- ↑ Filippopoulos T, Paula JS, Torun N, Hatton MP, Pasquale LR, Grosskreutz CL. (2008). „Periorbital changes associated with topical bimatoprost.”. Ophthalmology Plastic and Reconstructive Surgery 24 (4): 302–307. DOI:10.1097/IOP.0b013e31817d81df. PMID 18645437.
Literatura[uredi | uredi kod]
- Chen M, Cheng C, Chen Y, Chou C, Hsu W (2006). „Effects of bimatoprost 0.03% on ocular hemodynamics in normal tension glaucoma.”. J Ocul Pharmacol Ther 22 (3): 188–93. DOI:10.1089/jop.2006.22.188. PMID 16808680.
- Kruse P, Rieck P, Sherif Z, Liekfeld A (2006). „Cystoid macular edema in a pseudophakic patient after several glaucoma procedures. Is local therapy with bimatoprost the reason?”. Klinische Monatsblätter für Augenheilkunde 223 (6): 534–7. DOI:10.1055/s-2005-858992. PMID 16804825.
- Steinhäuser S (2006). „Decreased high-density lipoprotein serum levels associated with topical bimatoprost therapy.”. Optometry 77 (4): 177–9. DOI:10.1016/j.optm.2006.02.001. PMID 16567279.
- Park J, Cho HK, Moon JI (2011). „Changes to upper eyelid orbital fat from use of topical bimatoprost, travoprost, and latanoprost.”. Japanese Ophthalmological Society 55 (1): 22–27. DOI:10.1007/s10384-010-0904-z. PMID 21331688.
- Jayaprakasam A, Ghazi-Nouri S. (2010). „Periorbital fat atrophy - an unfamiliar side effect of prostaglandin analogues.”. Orbit 29 (6): 357–359. DOI:10.3109/01676830.2010.527028. PMID 21158579.
- Filippopoulos T, Paula JS, Torun N, Hatton MP, Pasquale LR, Grosskreutz CL. (2008). „Periorbital changes associated with topical bimatoprost.”. Ophthalmology Plastic and Reconstructive Surgery 24 (4): 302–307. DOI:10.1097/IOP.0b013e31817d81df. PMID 18645437.
Spoljašnje veze[uredi | uredi kod]
- Medical News Today: FDA Seizes $2 Million Of Cosmetic Eye Product Which Contains Drug Ingredient And Makes Unapproved Drug Claims. Christian Nordqvist. 18 November 2007
- Wired Science: FDA Seizes Cosmetic That Can Blind. Brandon Keim. November 19, 2007