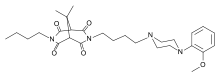
Agonisti :
Azapironi :
Alnespiron •
Binospiron •
Buspiron •
Enilospiron •
Eptapiron •
Gepiron •
Ipsapiron •
Perospiron •
Revospiron •
Tandospiron •
Tiospiron •
Umespiron •
Zalospiron ;
Antidepresivi :
Etoperidon •
Nefazodon •
Trazodon ;
Antipsihotici :
Aripiprazol •
Asenapin •
Klozapin •
Kvetiapin •
Ziprasidon ;
Ergolini :
Dihidroergotamin •
Ergotamin •
Lisurid •
Metisergid •
LSD ;
Triptamini :
5-CT •
5-MeO-DMT •
5-MT •
Bufotenin •
DMT •
Indorenat •
Psilocin •
Psilocibin ;
Drugi :
8-OH-DPAT •
Adatanserin •
Bay R 1531 •
Befiradol •
BMY-14802 •
Kanabidiol •
Dimemebfe •
Ebalzotan •
Eltoprazin •
F-11,461 •
F-12,826 •
F-13,714 •
F-14,679 •
F-15,063 •
F-15,599 •
Flesinoksan •
Flibanserin •
Lesopitron •
Lu AA21004 •
LY-293,284 •
LY-301,317 •
MKC-242 •
NBUMP •
Osemozotan •
Oksaflozan •
Pardoprunoks •
Piklozotan •
Rauvolscin •
Repinotan •
Roksindol •
RU-24,969 •
S 14,506 •
S-14,671 •
S-15,535 •
Sarizotan •
SSR-181,507 •
Sunepitron •
U-92,016-A •
Urapidil •
Vilazodon •
Ksaliproden •
Johimbin Antagonisti :
Antipsihotici :
Iloperidon •
Risperidon •
Sertindol ;
Beta blokatori :
Alprenolol •
Cianopindolol •
Jodocianopindolol •
Oksprenolol •
Pindobind •
Pindolol •
Propranolol •
Tertatolol ;
Drugi :
AV965 •
BMY-7,378 •
CSP-2503 •
Dotarizin •
Flopropion •
GR-46611 •
Isamoltan •
Lekozotan •
Metitepin/Metiotepin •
MPPF •
NAN-190 •
PRX-00023 •
Robalzotan •
S-15535 •
SB-649,915 •
SDZ 216-525 •
Spiperon •
Spiramid •
Spiroksatrin •
UH-301 •
WAY-100,135 •
WAY-100,635 •
Ksilamidin Agonisti :
Lisergamidi :
Dihidroergotamin •
Metisergid ;
Triptani :
Almotriptan •
Avitriptan •
Eletriptan •
Frovatriptan •
Naratriptan •
Rizatriptan •
Sumatriptan •
Zolmitriptan ;
Triptamini :
5-CT •
5-Etil-DMT •
5-MT •
5-(Noniloksi)triptamin ;
Drugi :
CP-135,807 •
CP-286,601 •
GR-46611 •
L-694,247 •
L-772,405 •
PNU-109,291 •
PNU-142,633 Antagonisti :
Lisergamidi :
Metergolin ;
Drugi :
Alniditan •
BRL-15,572 •
Elzasonan •
GR-127,935 •
Ketanserin •
LY-310,762 •
LY-367,642 •
LY-456,219 •
LY-456,220 •
Metitepin/Metiotepin •
Ritanserin •
Johimbin •
Ziprasidon
Agonisti :
Fenetilamini :
2C-B •
2C-E •
2C-I •
2C-T-2 •
2C-T-7 •
2C-T-21 •
DOB •
DOC •
DOI •
DOM •
MDA •
MDMA •
Meskalin ;
Piperazini :
Aripiprazol •
mCPP •
TFMPP ;
Triptamini :
5-CT •
5-MeO-α-ET •
5-MeO-α-MT •
5-MeO-DET •
5-MeO-DiPT •
5-MeO-DMT •
5-MeO-DPT •
5-MT •
α-ET •
α-Metil-5-HT •
α-MT •
Bufotenin •
DET •
DiPT •
DMT •
DPT •
Psilocin •
Psilocibin ;
Drugi :
A-372,159 •
AL-38022A •
CP-809,101 •
Dimemebfe •
Lorkaserin •
Medifoksamin •
MK-212 •
Org 12,962 •
ORG-37,684 •
Oksaflozan •
PNU-22394 •
Ro60-0175 •
Ro60-0213 •
Vabicaserin •
WAY-629 •
WAY-161,503 •
YM-348 Antagonisti :
Atipični antipsihotici :
Klozapin •
Iloperidon •
Melperon •
Olanzapin •
Paliperidon •
Pimozid •
Hetiapin •
Risperidon •
Sertindol •
Ziprasidon •
Zotepin ;
Tipični antipsihotici :
Hlorpromazin •
Loksapin •
Pipamperon ;
Antidepresivi :
Agomelatin •
Amitriptilin •
Amoksapin •
Aptazapin •
Etoperidon •
Fluoksetin •
Mianserin •
Mirtazapin •
Nefazodon •
Nortriptilin •
Trazodon ;
Drugi :
Adatanserin •
Cinanserin •
Ciproheptadin •
Deramciklan •
Dotarizin •
Eltoprazin •
Esmirtazapin •
FR-260,010 •
Ketanserin •
Ketotifen •
Latrepirdin •
Lu AA24530 •
Metitepin/Metiotepin •
Metisergid •
Pizotifen •
Ritanserin •
RS-102,221 •
S-14,671 •
SB-200,646 •
SB-206,553 •
SB-221,284 •
SB-228,357 •
SB-242,084 •
SB-243,213 •
SDZ SER-082 •
Ksilamidin
Agonisti :
Lisergamidi :
Dihidroergotamin •
Ergotamin •
Lisurid •
LSD •
Mesulergin •
Metergolin •
Metisergid ;
Triptamini :
2-Metil-5-HT •
5-BT •
5-CT •
5-MT •
Bufotenin •
E-6801 •
E-6837 •
EMD-386,088 •
EMDT •
LY-586,713 •
N -Metil-5-HT •
Triptamin ;
Drugi :
WAY-181,187 •
WAY-208,466 Antagonisti :
Antidepresanti :
Amitriptilin •
Amoksapin •
Klomipramin •
Doksepin •
Mianserin •
Nortriptilin ;
Atipični antipsihotici :
Aripiprazol •
Asenapin •
Klozapin •
Fluperlapin •
Iloperidon •
Olanzapin •
Tiospiron ;
Tipični antipsihotici :
Hlorpromazin •
Loksapin ;
Drugi :
BGC20-760 •
BVT-5182 •
BVT-74316 •
EGIS-12,233 •
GW-742,457 •
Ketanserin •
Latrepirdin •
Lu AE58054 •
Metitepin/Metiotepin •
MS-245 •
PRX-07034 •
Ritanserin •
Ro04-6790 •
Ro 63-0563 •
SB-258,585 •
SB-271,046 •
SB-357,134 •
SB-399,885 •
SB-742,457 Agonisti :
Lisergamidi :
LSD ;
Triptamini :
5-CT •
5-MT •
Bufotenin ;
Drugi :
8-OH-DPAT •
AS-19 •
Bifeprunoks •
LP-12 •
LP-44 •
RU-24,969 •
Sarizotan Antagonisti :
Lisergamidi :
2-Bromo-LSD •
Bromokriptin •
Dihidroergotamin •
Ergotamin •
Mesulergin •
Metergolin •
Metisergid ;
Antidepresanti :
Amitriptilin •
Amoksapin •
Klomipramin •
Imipramin •
Maprotilin •
Mianserin ;
Atipični antipsihotici :
Amisulprid •
Aripiprazol •
Klozapin •
Olanzapin •
Risperidon •
Sertindol •
Tiospiron •
Ziprasidon •
Zotepin ;
Tipični antipsihotici :
Hlorpromazin •
Loksapin ;
Drugi :
Butaklamol •
EGIS-12,233 •
Ketanserin •
LY-215,840 •
Metitepin/Metiotepin •
Pimozid •
Ritanserin •
SB-258,719 •
SB-258,741 •
SB-269,970 •
SB-656,104 •
SB-656,104-A •
SB-691,673 •
SLV-313 •
SLV-314 •
Spiperon •
SSR-181,507