Enoksacin
Prijeđi na navigaciju
Prijeđi na pretragu
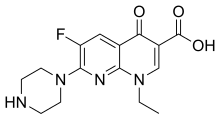
| |||
| (IUPAC) ime | |||
|---|---|---|---|
| 1-etil-6-fluoro-4-okso-7-(piperazin-1-il)-1,4-dihidro-1,8-naftiridin-3-karboksilna kiselina | |||
| Klinički podaci | |||
| AHFS/Drugs.com | Monografija | ||
| MedlinePlus | a601013 | ||
| Identifikatori | |||
| CAS broj | 74011-58-8 | ||
| ATC kod | J01MA04 | ||
| PubChem[1][2] | 3229 | ||
| DrugBank | DB00467 | ||
| ChemSpider[3] | 3116 | ||
| UNII | 325OGW249P | ||
| KEGG[4] | D00310 | ||
| ChEBI | CHEBI:157175 | ||
| ChEMBL[5] | CHEMBL826 | ||
| Hemijski podaci | |||
| Formula | C15H17FN4O3 | ||
| Mol. masa | 320,319 g/mol | ||
| SMILES | eMolekuli & PubHem | ||
| |||
| Farmakoinformacioni podaci | |||
| Trudnoća | ? | ||
| Pravni status | |||
| Način primene | Oralno | ||
Enoksacin (Almitil, Bactidan, Bactidron, Comprecin, Enoksetin, Enoxen, Enroxil, Enoxin, Enoxor, Flumark, Penetrex, Gyramid, Vinone) je oralni fluorohinolonski antibakterijski agens širokog spektra koji se koristi u tretmanu infekcija urinarnog trakta i gonoreja. Insomnija je česta nuspojava pri upotrebi ovog leka.[6][7]
Nedavno je pokazano da ova supstanca može da inhibira kancer.[8]
Reference[uredi | uredi kod]
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519.
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining”. J Cheminform 2 (1): 3. DOI:10.1186/1758-2946-2-3. PMID 20331846.
- ↑ Joanne Wixon, Douglas Kell (2000). „Website Review: The Kyoto Encyclopedia of Genes and Genomes — KEGG”. Yeast 17 (1): 48–55. DOI:10.1002/(SICI)1097-0061(200004)17:1<48::AID-YEA2>3.0.CO;2-H.
- ↑ Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B, Overington JP. (2012). „ChEMBL: a large-scale bioactivity database for drug discovery”. Nucleic Acids Res 40 (Database issue): D1100-7. DOI:10.1093/nar/gkr777. PMID 21948594.
- ↑ Rafalsky, V.; Andreeva, I.; Rjabkova, E.; Rafalsky, Vladimir V (2006). Rafalsky, Vladimir V. ur. „Quinolones for uncomplicated acute cystitis in women”. Cochrane Database Syst Rev 3: CD003597. DOI:10.1002/14651858.CD003597.pub2. PMID 16856014.
- ↑ Mogabgab, WJ. (Dec 1991). „Recent developments in the treatment of sexually transmitted diseases”. Am J Med 91 (6A): 140S–144S. DOI:10.1016/0002-9343(91)90327-T. PMID 1767802.
- ↑ Sonia Melo, Alberto Villanueva, Catia Moutinho, Veronica Davalos, Riccardo Spizzo, Cristina Ivan, Simona Rossi, Fernando Setien, Oriol Casanovas, Laia Simo-Riudalbas, Javier Carmona, Jordi Carrere, August Vidal, Alvaro Aytes, Sara Puertas, Santiago Ropero, Raghu Kalluri, Carlo M. Croce, George A. Calin, Manel Estellera (2011). „Small molecule enoxacin is a cancer-specific growth inhibitor that acts by enhancing TAR RNA-binding protein 2-mediated microRNA processing”. PNAS. DOI:10.1073/pnas.1014720108. Arhivirano iz originala na datum 2014-08-26. Pristupljeno 2014-04-05.
Literatura[uredi | uredi kod]
- Patel SS, Spencer CM (January 1996). „Enoxacin: a reappraisal of its clinical efficacy in the treatment of genitourinary tract infections”. Drugs 51 (1): 137–60. PMID 8741236..